

AVENUE THERAPEUTICS, INC. ⎸ NASDAQ: ATXI ⎸ OCTOBER 2022 Corporate Overview Issuer Free Writing Prospectus Filed Pursuant to Rule 433 Registration Statement No. 333 - 267206 (Preliminary Prospectus dated October 6, 2022)

2 Notice & Disclaimers Free Writing Prospectus This free writing prospectus relates to the proposed public offering of securities of Avenue Therapeutics, Inc. (the “Company ”), which is being registered with the U.S. Securities and Exchange Commission (the “SEC”) on a Registration Statement on Form S - 1 (No. 333 - 267206) (the as amended, “Registration Statement”). This free writing prospectus should be read together with the preliminary prospectus dated October 6, 2022 included within the Registration Statement pursuant to the Securities Act of 193 3, as amended, which has preceded this free writing prospectus and can be accessed through the following link: https://www.sec.gov/Archives/edgar/data/1644963/000110465922105952/tm2224752d2_s1.htm . The Company has filed a registration statement (including a preliminary prospectus) with the SEC for the offering to which th is communication relates. Before you invest, you should read the registration statement (including the preliminary prospectus) and other documents the Company has filed with the SEC for more complete information about the Company and this offering. You may get these documents for free by visiting EDGAR on the SEC Web site at www.sec.gov or on the Company’s Investor Relations web site at http://ir.avenuetx.com. Alternatively, the Company, any underwriter or any dealer participating in the offering will arrange to send you the prospectus if you request it by call ing the Prospectus Department at Aegis Capital Corp. at 1 (646) 502 - 2418. Cautionary Note Regarding Forward - Looking Statements This presentation contains predictive or “forward - looking statements” within the meaning of the Private Securities Litigation Re form Act of 1995. All statements other than statements of current or historical fact contained in this presentation, including statements that express our intentions, plans, objectives, beliefs, ex pectations, strategies, predictions or any other statements relating to our future activities or other future events or conditions are forward - looking statements. The words “anticipate,” “believe,” “conti nue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “will,” “should,” “would” and similar expressions are intended to identify forward - looking statements. These statemen ts are based on current expectations, estimates and projections made by management about our business, our industry and other conditions affecting our financial condition, results of operations or business prospects. These statements are not guarantees of future performance and involve risks, uncertainties and assumptions that are difficult to predict. Therefore, actual outcomes and re sul ts may differ materially from what is expressed or forecasted in, or implied by, the forward - looking statements due to numerous risks and uncertainties. Factors that could cause such outcomes and r esults to differ include, but are not limited to, risks and uncertainties arising from: expectations for increases or decreases in expenses; expectations for the clinical and pre - clinical development, manufacturing, regulatory approval, and commercialization of our pharmaceutical product candidate or any other products we may acquire or in - license; our use of clinica l research centers and other contractors; expectations for incurring capital expenditures to expand our research and development and manufacturing capabilities; expectations for generating reven ue or becoming profitable on a sustained basis; expectations or ability to enter into marketing and other partnership agreements; expectations or ability to enter into product acquisition a nd in - licensing transactions; expectations or ability to build our own commercial infrastructure to manufacture, market and sell our product candidate; acceptance of our products by doctors, patie nts or payors; our ability to compete against other companies and research institutions; our ability to secure adequate protection for our intellectual property; our ability to attract and re tai n key personnel; availability of reimbursement for our products; estimates of the sufficiency of our existing cash and cash equivalents and investments to finance our operating requirements, including ex pec tations regarding the value and liquidity of our investments; the volatility of our stock price; expected losses expectations for future capital requirements; uncertainty surrounding the Baer gic Bio acquisition; and those risks discussed in our filings which we make with the SEC. Any forward - looking statements speak only as of the date on which they are made, and we undertake no obligation to publicly update or revise any forward - looking statements to reflect events or circumstances that may arise after the date of this presentation, except as required by applicable law. Inv est ors should evaluate any statements made by us in light of these important factors.
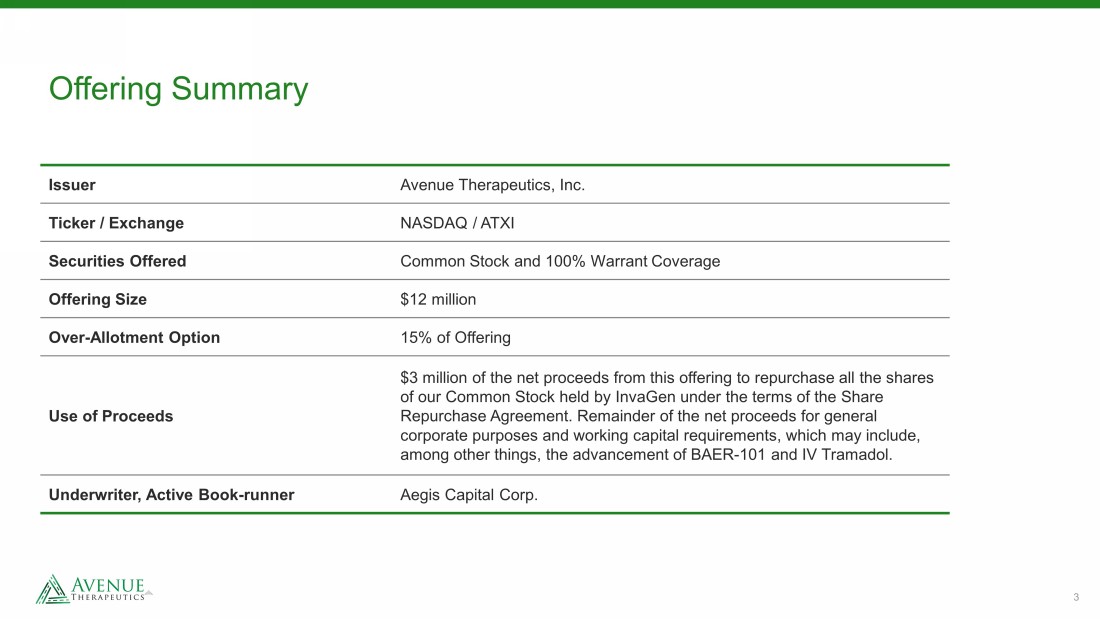
3 Offering Summary Issuer Avenue Therapeutics, Inc. Ticker / Exchange NASDAQ / ATXI Securities Offered Common Stock and 100% Warrant Coverage Offering Size $12 million Over - Allotment Option 15% of Offering Use of Proceeds $3 million of the net proceeds from this offering to repurchase all the shares of our Common Stock held by InvaGen under the terms of the Share Repurchase Agreement. Remainder of the net proceeds for general corporate purposes and working capital requirements, which may include, among other things, the advancement of BAER - 101 and IV Tramadol. Underwriter, Active Book - runner Aegis Capital Corp.
4 Executive Summary Avenue Therapeutics is a specialty pharmaceutical company that seeks to develop and commercialize therapies to treat central nervous system (CNS) conditions Our portfolio will soon include two clinical stage programs that we believe each have a significant market potential – IV Tramadol (Phase 3) for acute postoperative surgical pain – BAER - 101* (Phase 1b) for epilepsy and acute anxiety Our clinical development programs are designed to meet near - term milestones that could build near - term value Potential to continue to expand pipeline through additional acquisitions in the rare / CNS disease space 4 CONFIDENTIAL * Pending close of Share Contribution Agreement with Fortress Biotech, Inc. for Baergic Bio, Inc.
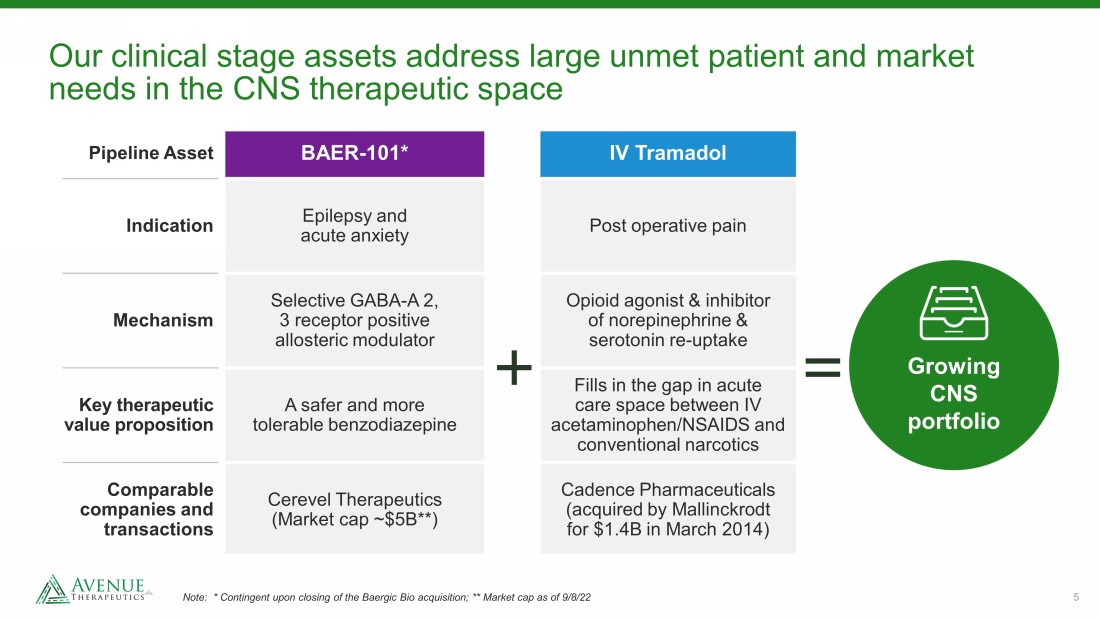
5 Our clinical stage assets address large unmet patient and market needs in the CNS therapeutic space Note: * Contingent upon closing of the Baergic Bio acquisition; ** Market cap as of 9/8/22 Pipeline Asset BAER - 101* IV Tramadol Indication Epilepsy and acute anxiety Post operative pain Mechanism Selective GABA - A 2, 3 receptor positive allosteric modulator Opioid agonist & inhibitor of norepinephrine & serotonin re - uptake Key therapeutic value proposition A safer and more tolerable benzodiazepine Fills in the gap in acute care space between IV acetaminophen/NSAIDS and conventional narcotics Comparable companies and transactions Cerevel Therapeutics (Market cap ~$5B**) Cadence Pharmaceuticals (acquired by Mallinckrodt for $1.4B in March 2014) Growing CNS portfolio
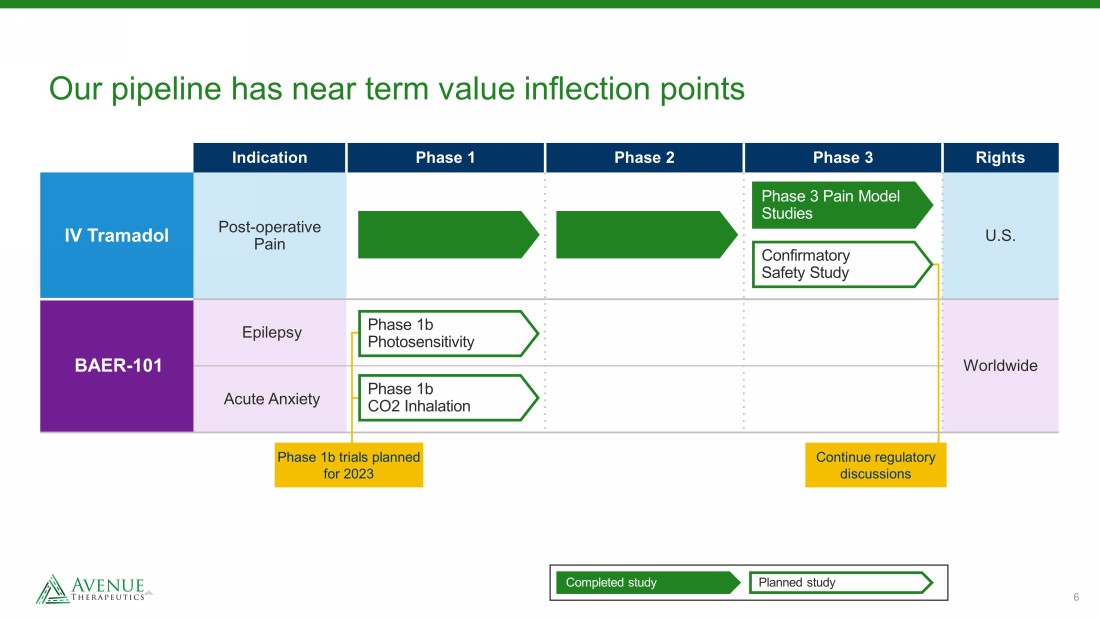
6 Our pipeline has near term value inflection points Indication Phase 1 Phase 2 Phase 3 Rights IV Tramadol Post - operative Pain U.S. BAER - 101 Epilepsy Worldwide Acute Anxiety Continue regulatory discussions Completed study Planned study Phase 3 Pain Model Studies Confirmatory Safety Study Phase 1b trials planned for 2023 Phase 1b Photosensitivity Phase 1b CO2 Inhalation
7 BAER - 101 IV Tramadol Acquire CNS / Rare Disease Assets 1 2 3

8 Epilepsy Anti - Convulsant Effects Acute Anxiety Conditions (e.g., Panic Disorder) Anxiolytic Effects BAER - 101 is being developed for epilepsy and acute anxiety BAER - 101
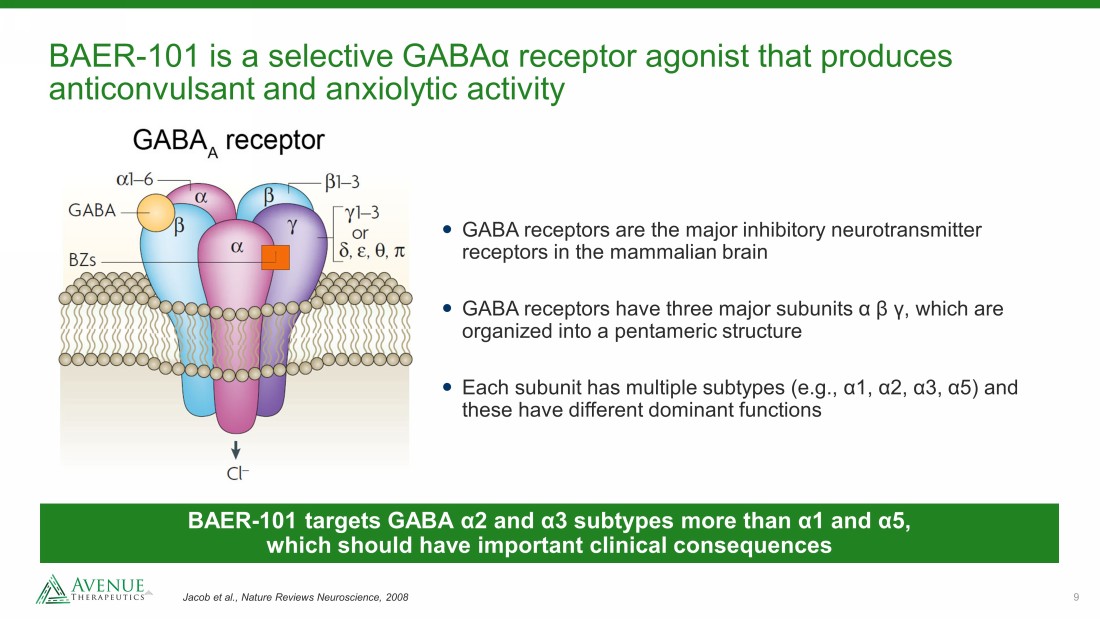
9 BAER - 101 is a selective GABA α receptor agonist that produces anticonvulsant and anxiolytic activity Jacob et al., Nature Reviews Neuroscience, 2008 GABA receptors are the major inhibitory neurotransmitter receptors in the mammalian brain GABA receptors have three major subunits α β γ , which are organized into a pentameric structure Each subunit has multiple subtypes (e.g., α 1, α 2, α 3, α 5) and these have different dominant functions BAER - 101 targets GABA α2 and α3 subtypes more than α1 and α5, which should have important clinical consequences
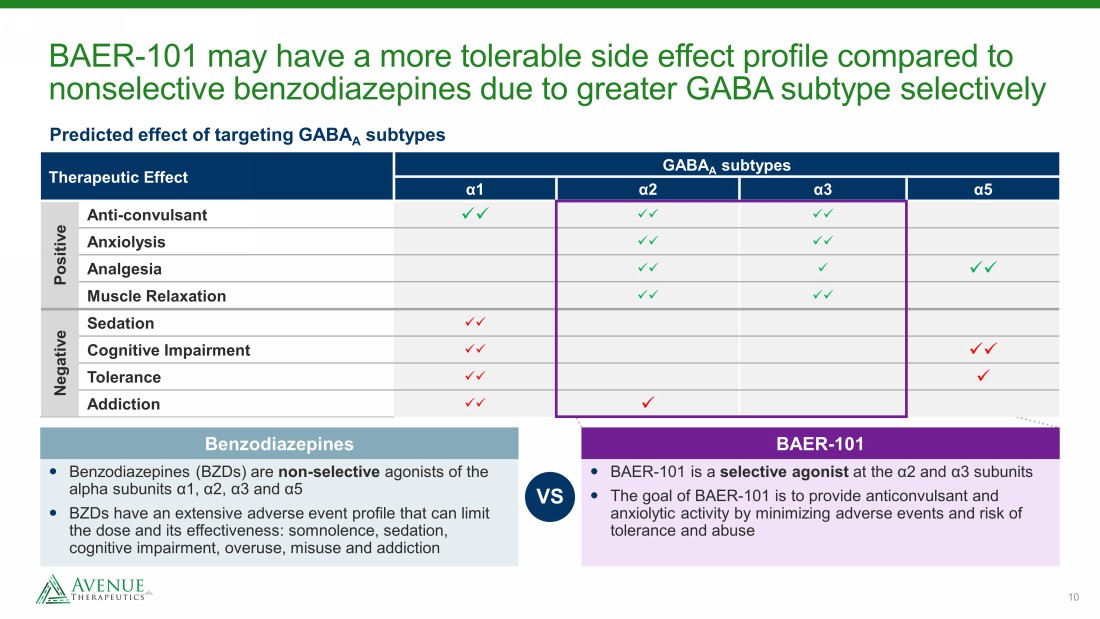
10 BAER - 101 may have a more tolerable side effect profile compared to nonselective benzodiazepines due to greater GABA subtype selectively Therapeutic Effect GABA A subtypes α 1 α 2 α 3 α 5 Positive Anti - convulsant xx xx xx Anxiolysis xx xx Analgesia xx x xx Muscle Relaxation xx xx Negative Sedation xx Cognitive Impairment xx xx Tolerance xx x Addiction xx x Predicted effect of targeting GABA A subtypes BAER - 101 is a selective agonist at the α 2 and α 3 subunits The goal of BAER - 101 is to provide anticonvulsant and anxiolytic activity by minimizing adverse events and risk of tolerance and abuse BAER - 101 Benzodiazepines (BZDs) are non - selective agonists of the alpha subunits α 1, α 2, α 3 and α 5 BZDs have an extensive adverse event profile that can limit the dose and its effectiveness: somnolence, sedation, cognitive impairment, overuse, misuse and addiction Benzodiazepines VS

11 Epilepsy patients are prescribed benzodiazepines to control seizures, but have significant unmet needs for therapies with improved safety profiles Source: CDC.gov; Kalilani L et al. The epidemiology of drug - resistant epilepsy: A systematic review and meta - analysis. Epilepsia . 2018 Dec Disease Epilepsy is a chronic disease that manifests as recurrent seizures from abnormal electrical discharge in the brain Affects approximately 65M patients worldwide Treatment Unmet Need Standard of care is the use of one or more anti - epileptic drugs (AED); Benzodiazepines (BZDs) are a class of AED that are used to treat seizures, but are generally not used as daily medication due to side effects ~50% of epilepsy patients fail 1 st line treatments and ~30% of patients are not well managed with any antiseizure medications BZDs are effective for seizures, but not well tolerated due to significant side effects including sedation, cognitive impairment, ataxia and can develop tolerance and addiction over time

12 Acute anxiety patients also utilize benzodiazepines today and have significant unmet need for therapies with improved safety profiles Disease Panic disorder is a common form of an acute anxiety disorder manifesting as frequent panic attacks unrelated to specific situations Manifests as intense episodes of apprehension, terror, feelings of impending doom and intense urge to flee Affects approximately 6.8M patients in the U.S. Treatment Unmet Need Panic disorder is treated with a combination of cognitive behavioral therapy and benzodiazepines (BZDs), tricyclics, selective serotonin reuptake inhibitors (SSRIs), and serotonin - norepinephrine reuptake inhibitors (SNRIs) BZDs are effective for panic disorder but not well tolerated due to sedation, cognitive impairment, ataxia and can develop tolerance and addiction over time Source: ADAA.org
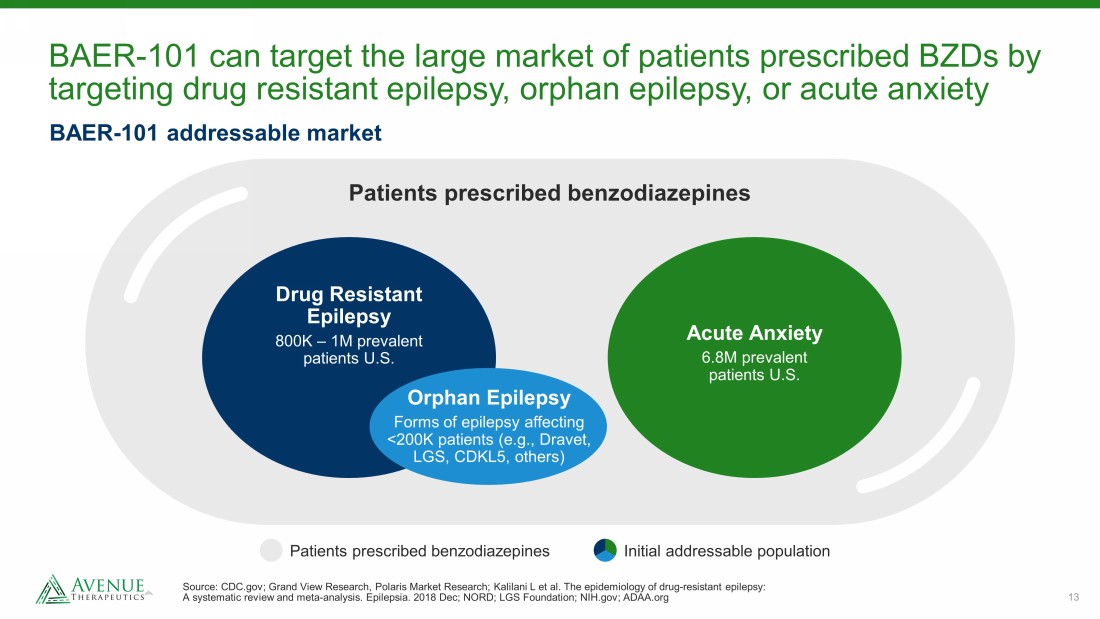
13 BAER - 101 can target the large market of patients prescribed BZDs by targeting drug resistant epilepsy, orphan epilepsy, or acute anxiety Source: CDC.gov; Grand View Research, Polaris Market Research; Kalilani L et al. The epidemiology of drug - resistant epilepsy: A systematic review and meta - analysis. Epilepsia. 2018 Dec; NORD; LGS Foundation; NIH.gov; ADAA.org Patients prescribed benzodiazepines Drug Resistant Epilepsy 800K – 1M prevalent patients U.S. Orphan Epilepsy Forms of epilepsy affecting <200K patients (e.g., Dravet, LGS, CDKL5, others) Acute Anxiety 6.8M prevalent patients U.S. BAER - 101 addressable market Patients prescribed benzodiazepines Initial addressable population
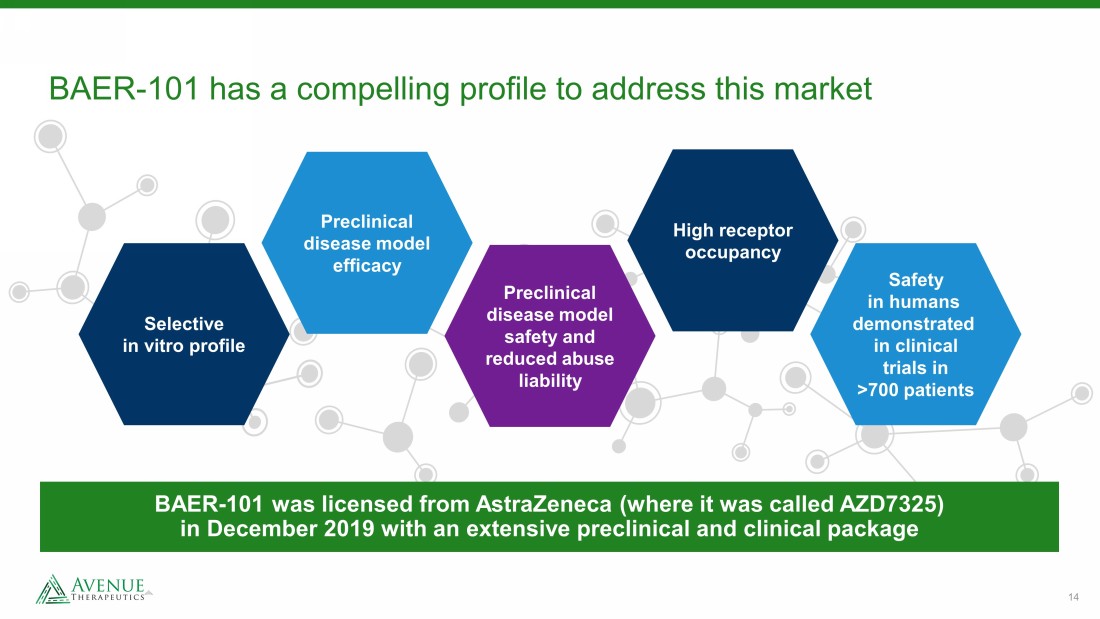
14 BAER - 101 has a compelling profile to address this market Safety in humans demonstrated in clinical trials in >700 patients Preclinical disease model safety and reduced abuse liability Preclinical disease model efficacy Selective in vitro profile High receptor occupancy BAER - 101 was licensed from AstraZeneca (where it was called AZD7325) in December 2019 with an extensive preclinical and clinical package
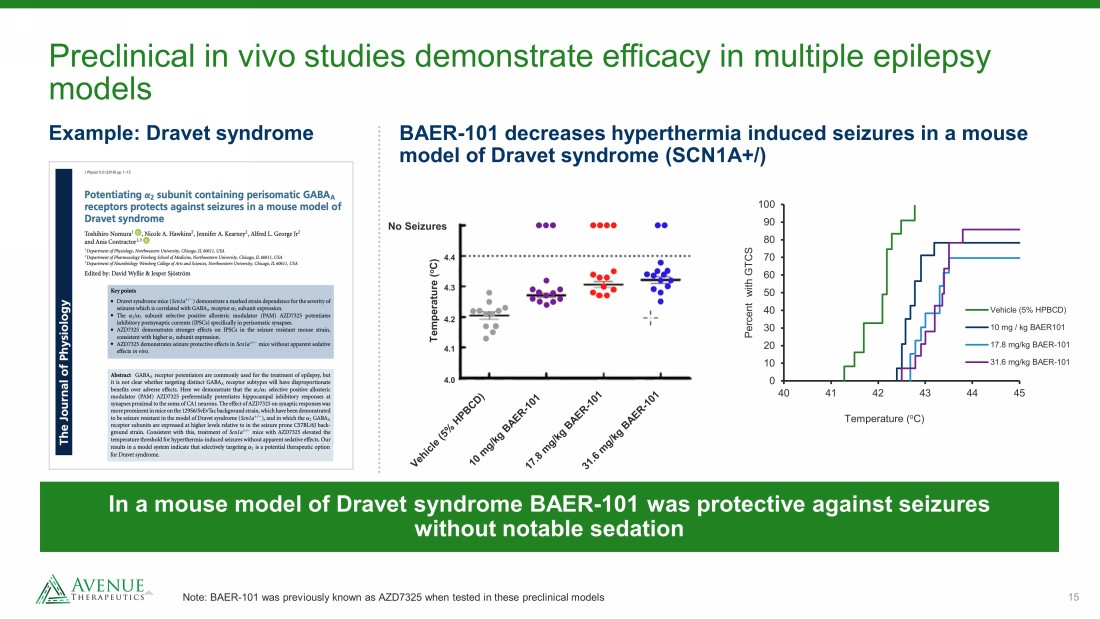
15 Preclinical in vivo studies demonstrate efficacy in multiple epilepsy models Note: BAER - 101 was previously known as AZD7325 when tested in these preclinical models BAER - 101 decreases hyperthermia induced seizures in a mouse model of Dravet syndrome (SCN1A+/) In a mouse model of Dravet syndrome BAER - 101 was protective against seizures without notable sedation Example: Dravet syndrome Temperature ( o C) No Seizures 4.0 4.2 4.1 4.3 4.4 0 10 20 30 40 50 60 70 80 90 100 40 41 42 43 44 45 Percent with GTCS Temperature ( o C) Vehicle (5% HPBCD) 10 mg / kg BAER101 17.8 mg/kg BAER-101 31.6 mg/kg BAER-101
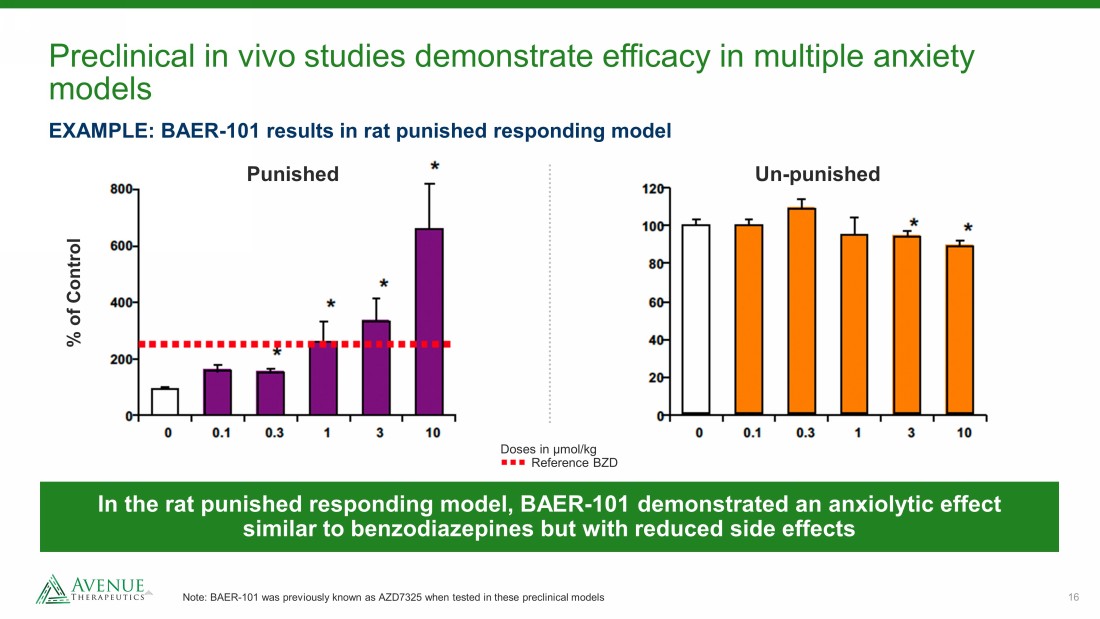
16 Preclinical in vivo studies demonstrate efficacy in multiple anxiety models Note: BAER - 101 was previously known as AZD7325 when tested in these preclinical models EXAMPLE: BAER - 101 results in rat punished responding model Punished Un - punished % of Control In the rat punished responding model, BAER - 101 demonstrated an anxiolytic effect similar to benzodiazepines but with reduced side effects Doses in µmol/kg Reference BZD

17 AstraZeneca completed 10 clinical studies and demonstrated safety across trials BAER - 101/AZD7325 was tested in over 700 subjects (healthy volunteers and patients) Side effects were mild or moderate with the most common side effects being dizziness and somnolence In two Phase 2 studies, BAER - 101 was tested in patients with generalized anxiety disorder (GAD), but missed the primary endpoint – A sub analysis of the data with removal of dropouts and non - compliant patients (as measured by drug plasma levels), showed: • a dose - related anxiolytic signal • a correlation between average exposure and efficacy – Further, Cerevel’s darigabat (a similar molecule) also missed the primary endpoint in the truncated GAD study and showed promising results in two Phase 1b studies in epilepsy and acute anxiety BAER - 101 was also tested in a human abuse liability study where risk abuse with BAER - 101 appeared lower than lorazepam (a BZD)
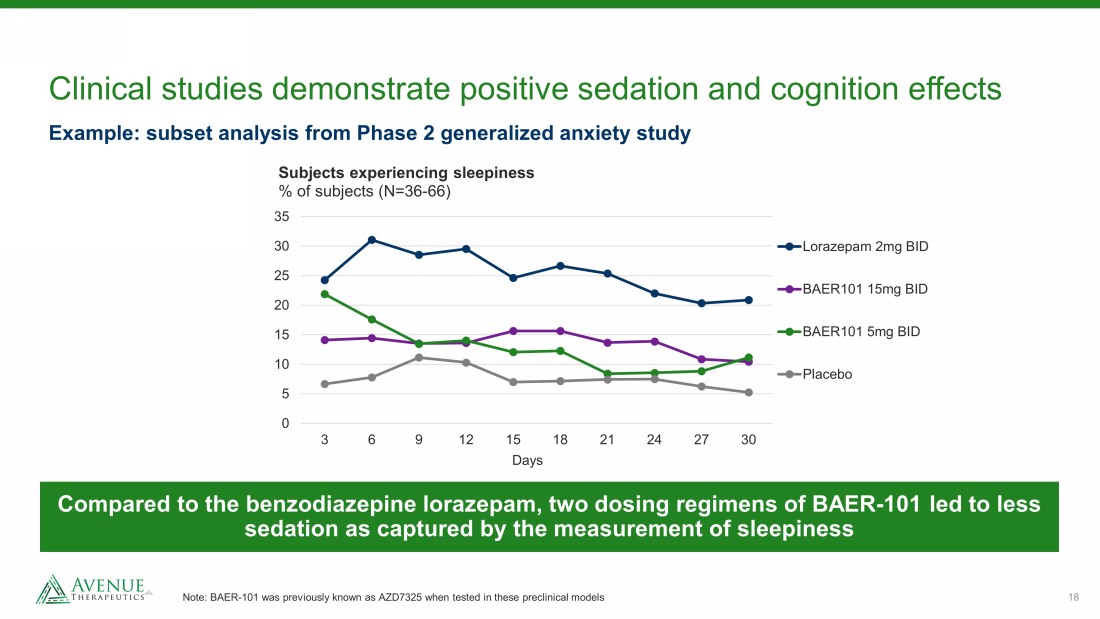
18 Clinical studies demonstrate positive sedation and cognition effects Note: BAER - 101 was previously known as AZD7325 when tested in these preclinical models 0 5 10 15 20 25 30 35 3 6 9 12 15 18 21 24 27 30 Subjects experiencing sleepiness % of subjects (N=36 - 66) Lorazepam 2mg BID BAER101 15mg BID BAER101 5mg BID Placebo Example: subset analysis from Phase 2 generalized anxiety study Compared to the benzodiazepine lorazepam, two dosing regimens of BAER - 101 led to less sedation as captured by the measurement of sleepiness Days
19 We plan to initiate two Phase 1b studies that we expect to translate well into later development programs Epilepsy: Photosensitivity Study Acute Anxiety: Hypercapnia CO2 Inhalation Model The epilepsy photosensitivity model is a clinical translational model that provides proof - of - principle for antiepileptic activity in early clinical development Testing new antiepileptic drugs in this clinical model can provide data that translates well into larger and other epilepsy populations The CO2 inhalation challenge is a clinical translational model well - established in both healthy volunteers and in patients with panic disorder that provides proof - of - principle for anxiolytic activity in early clinical development The model is sensitive to drugs used to treat anxiety disorders (including benzodiazepines & SSRIs) and emerging new treatments with novel mechanisms WARNING! The following contains bright, flashing lights and/or imagery that may cause discomfort and/or seizures for those with photosensitivity epilepsy. Viewer discretion is advised.
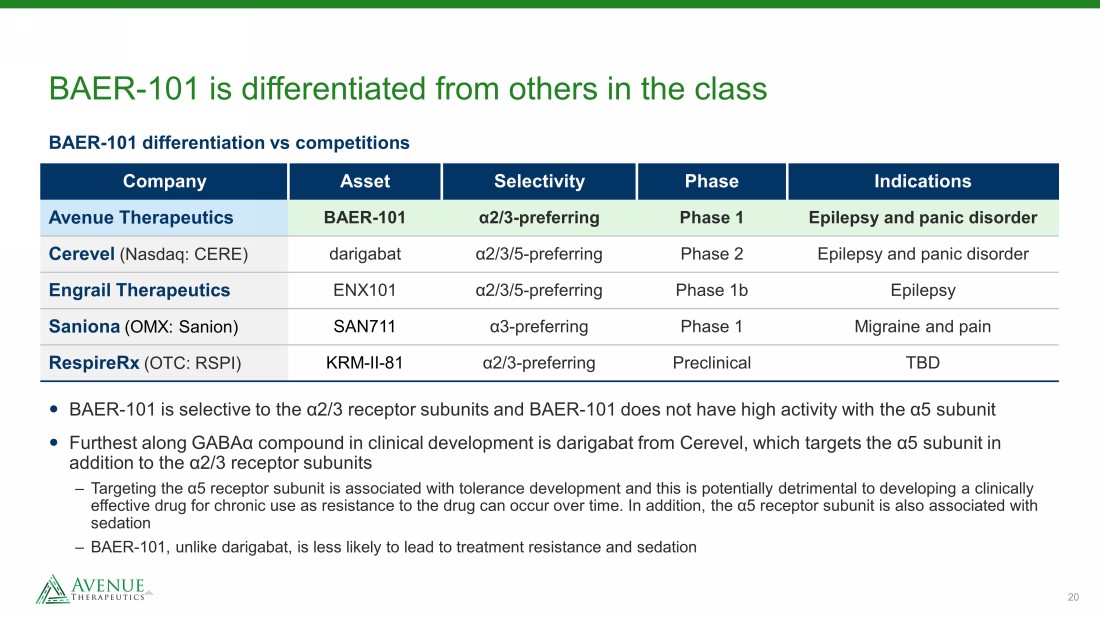
20 BAER - 101 is differentiated from others in the class Company Asset Selectivity Phase Indications Avenue Therapeutics BAER - 101 α 2/3 - preferring Phase 1 Epilepsy and panic disorder Cerevel (Nasdaq: CERE) darigabat α 2/3/5 - preferring Phase 2 Epilepsy and panic disorder Engrail Therapeutics ENX101 α 2/3/5 - preferring Phase 1b Epilepsy Saniona (OMX: Sanion) SAN711 α 3 - preferring Phase 1 Migraine and pain RespireRx (OTC: RSPI) KRM - II - 81 α 2/3 - preferring Preclinical TBD BAER - 101 differentiation vs competitions BAER - 101 is selective to the α2/3 receptor subunits and BAER - 101 does not have high activity with the α5 subunit Furthest along GABAα compound in clinical development is darigabat from Cerevel , which targets the α5 subunit in addition to the α2/3 receptor subunits – Targeting the α5 receptor subunit is associated with tolerance development and this is potentially detrimental to developing a c linically effective drug for chronic use as resistance to the drug can occur over time. In addition, the α5 receptor subunit is also as soc iated with sedation – BAER - 101, unlike darigabat , is less likely to lead to treatment resistance and sedation
21 BAER - 101 IV Tramadol Acquire CNS / Rare Disease Assets 1 2 3
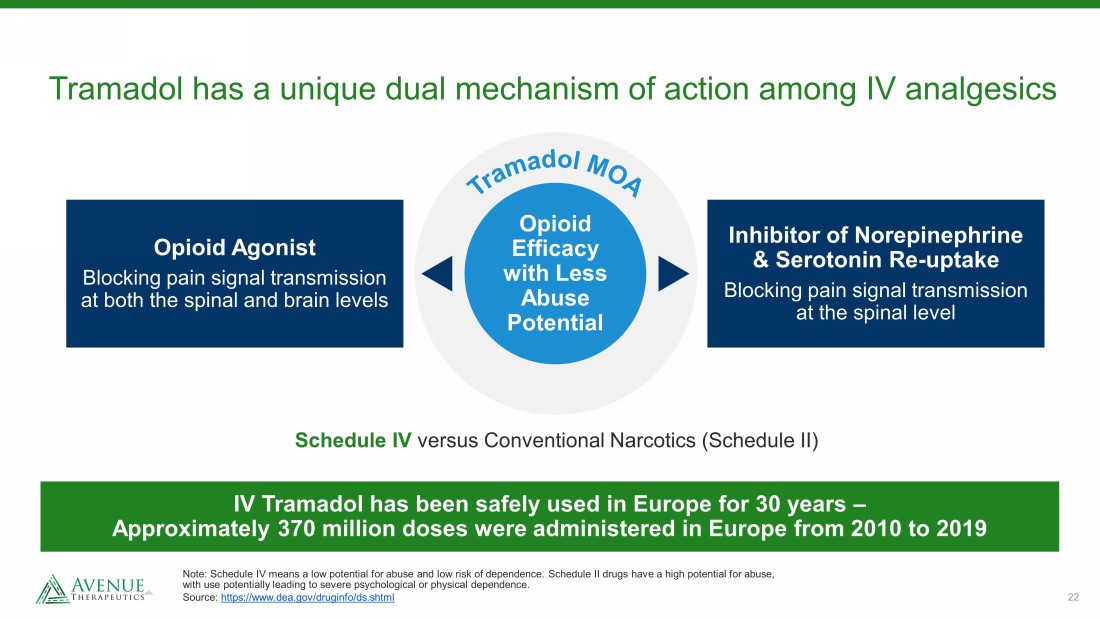
22 Tramadol has a unique dual mechanism of action among IV analgesics Note: Schedule IV means a low potential for abuse and low risk of dependence. Schedule II drugs have a high potential for abu se, with use potentially leading to severe psychological or physical dependence. Source: https://www.dea.gov/druginfo/ds.shtml Opioid Efficacy with Less Abuse Potential Schedule IV versus Conventional Narcotics (Schedule II) IV Tramadol has been safely used in Europe for 30 years – Approximately 370 million doses were administered in Europe from 2010 to 2019 Opioid Agonist Blocking pain signal transmission at both the spinal and brain levels Inhibitor of Norepinephrine & Serotonin Re - uptake Blocking pain signal transmission at the spinal level
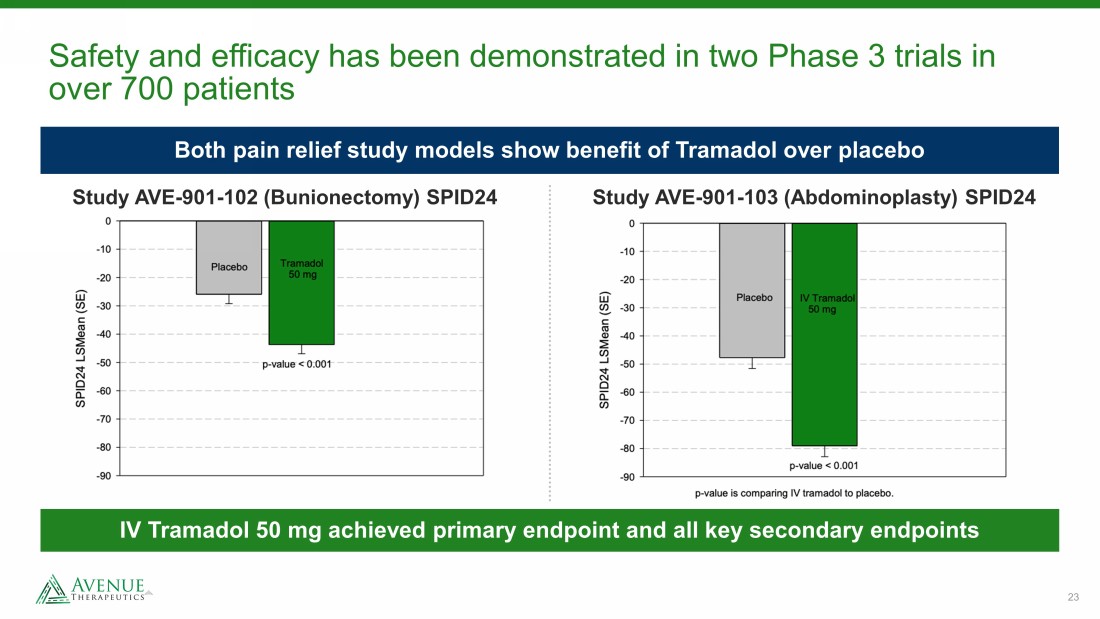
23 Safety and efficacy has been demonstrated in two Phase 3 trials in over 700 patients Both pain relief study models show benefit of Tramadol over placebo Study AVE - 901 - 103 (Abdominoplasty) SPID24 Study AVE - 901 - 102 (Bunionectomy) SPID24 IV Tramadol 50 mg achieved primary endpoint and all key secondary endpoints
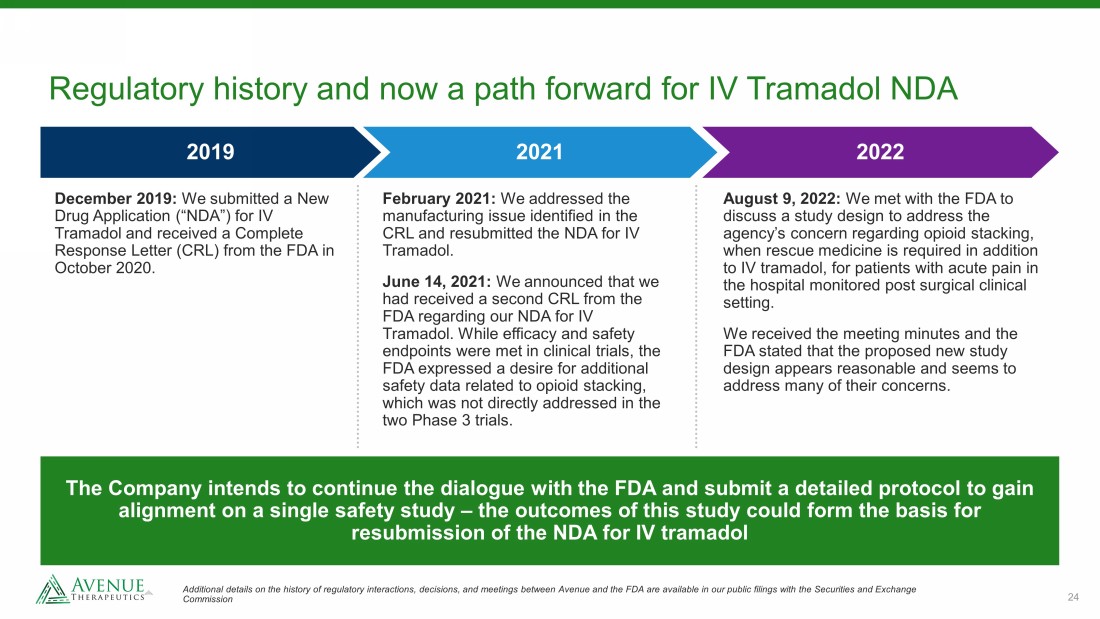
24 Regulatory history and now a path forward for IV Tramadol NDA The Company intends to continue the dialogue with the FDA and submit a detailed protocol to gain alignment on a single safety study – the outcomes of this study could form the basis for resubmission of the NDA for IV tramadol 2022 2019 2021 December 2019: We submitted a New Drug Application (“NDA”) for IV Tramadol and received a Complete Response Letter (CRL) from the FDA in October 2020. February 2021: We addressed the manufacturing issue identified in the CRL and resubmitted the NDA for IV Tramadol. June 14, 2021: We announced that we had received a second CRL from the FDA regarding our NDA for IV Tramadol. While efficacy and safety endpoints were met in clinical trials, the FDA expressed a desire for additional safety data related to opioid stacking, which was not directly addressed in the two Phase 3 trials. August 9, 2022: We met with the FDA to discuss a study design to address the agency’s concern regarding opioid stacking, when rescue medicine is required in addition to IV tramadol, for patients with acute pain in the hospital monitored post surgical clinical setting. We received the meeting minutes and the FDA stated that the proposed new study design appears reasonable and seems to address many of their concerns. Additional details on the history of regulatory interactions, decisions, and meetings between Avenue and the FDA are availabl e i n our public filings with the Securities and Exchange Commission
25 BAER - 101 IV Tramadol Acquire CNS / Rare Disease Assets 1 2 3
26 We are searching the world for first rate CNS / rare disease assets with these characteristics Feasible Clinical Development Programs with Reasonable Costs Imaging and Biomarker Driven Endpoints Clinical Stage with Human Data 26 CONFIDENTIAL

27 27 Avenue Company Overview

28 We are led by an experienced management team and board of directors Lindsay Rosenwald MD CEO, Fortress Biotech Jay Kranzler MD PhD Partner, Jay D Kranzler Consulting Neil Herskowitz Founder, ReGen Capital Curtis Oltmans Chief Legal Officer, Fulcrum Therapeutics Faith Charles Partner, Thompson Hine LLP E. Garrett Ingram President & CEO, Cipla Therapeutics, Inc. Jaideep Gogtay, MD CMO, Cipla Ltd. Management Board of Directors Alexandra MacLean MD CEO David Jin Interim CFO Michael Ryan VP Clinical Operations & Program Management

29 IP / Exclusivity BAER - 101 Two issued US patents and related foreign patents in European States (CH, DE, ES, FR, GB, IT and SE), China, Canada and Japan for composition of matter with expiry date of December 2026 – Eligible for patent term extension (expected 5 years) – Potential for Orphan Drug Designation for rare epilepsies (7 years market exclusivity) Two issued US patents for method of use in a childhood development disorder with expiry date of January 2036 Additional IP under development for specific indication needs IV Tramadol Proprietary dosing regimen is patent - protected in the US until 2036, excluding patent term extension Portfolio also includes drug patents covering combinations
30 Executive Summary Avenue Therapeutics is a specialty pharmaceutical company that seeks to develop and commercialize therapies to treat central nervous system (CNS) conditions Our portfolio will soon include two clinical stage programs that we believe each have a significant market potential – IV Tramadol (Phase 3) for acute postoperative surgical pain – BAER - 101* (Phase 1b) for epilepsy and acute anxiety Our clinical development programs are designed to meet near - term milestones that could build near - term value Potential to continue to expand pipeline through additional acquisitions in the rare / CNS disease space 30 CONFIDENTIAL * Pending close of Share Contribution Agreement with Fortress Biotech, Inc. for Baergic Bio, Inc.