
Filed Pursuant to Rule 424(b)(4)
Registration No. 333-267206
Prospectus
2,652,065 Units, each consisting of one Share of Common Stock and one Warrant to purchase Shares of Common Stock
and
984,300 Pre-Funded Units, each consisting of one Pre-funded Warrant to purchase Shares of Common Stock and one Warrant to purchase Shares of Common Stock

We are offering 2,652,065 units, each consisting of one share of our common stock, par value $0.0001 per share (“Common Stock”), and one warrant to purchase one share of our Common Stock in a firm commitment underwritten public offering.
We are also offering to those purchasers whose purchase of units in this offering would otherwise result in such purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock immediately following the consummation of this offering, 984,300 pre-funded units in lieu of units that would otherwise result in such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock. Each pre-funded unit consists of one pre-funded warrant to purchase one share of common stock and one warrant to purchase one share of common stock. The purchase price of each pre-funded unit will be equal to the price per unit being sold to the public in this offering, minus $0.0001, and the exercise price of each pre-funded warrant included in the pre-funded units will be $0.0001 per share. The pre-funded warrants included in the pre-funded units will be immediately exercisable and may be exercised at any time until all of the pre-funded warrants are exercised in full. The warrant included in the pre-funded unit is in the same form as the warrant included in the unit.
The units and the pre-funded units will not be issued or certificated. The shares of common stock or pre-funded warrants, as the case may be, and the accompanying warrants can only be purchased together in this offering, but the securities contained in the units or pre-funded units will be immediately separable upon issuance and will be issued separately. The shares of common stock issuable from time to time upon exercise of the warrants and the pre-funded warrants are also being offered by this prospectus.
The share and per share information in this prospectus reflects a one-for-fifteen reverse stock split of the outstanding Common Stock of the Company, which became effective on September 22, 2022.
Our Common Stock is quoted for trading under the symbol “ATXI” on the Nasdaq Capital Market. On October 6, 2022, the closing price of our Common Stock was $6.29 per share. There is no established public trading market for the warrants or the pre-funded warrants, and we do not expect such a market to develop.
We are an “emerging growth company” as defined under the federal securities laws and, as such, have elected to comply with certain reduced public company reporting requirements for this prospectus and our other filings with the Securities and Exchange Commission.
Investing in our securities involves risks that are described in the “Risk Factors” section beginning on page 11 of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of the securities to be issued under this prospectus or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Per Unit | Per Pre- Funded Unit | Total(2) | ||||||||||
| Public Offering Price | $ | 3.30 | $ | 3.2999 | $ | 12,000,004.50 | ||||||
| Underwriting discounts and commissions(1) | $ | 0.2805 | $ | 0.2805 | $ | 1,020,000.38 | ||||||
| Proceeds, before expenses, to us | $ | 3.0195 | $ | 3.0194 | $ | 10,980,004.12 | ||||||
| (1) | See “Underwriting” for additional disclosure regarding underwriting compensation. |
| (2) | Assumes no exercise of the underwriter’s over-allotment option to purchase additional securities granted to the underwriter as described below. |
We have granted the underwriter a 45-day option to purchase up to an aggregate of 545,454 additional shares of Common Stock, additional pre-funded units and/or additional warrants from us in any combination thereof, representing 15% of the securities sold in the offering, solely to cover over-allotments, if any, at the public offering price per share, per pre-funded warrant and per warrant, respectively.
The underwriter expects to deliver the securities against payment on or about October 11, 2022.
AEGIS CAPITAL CORP.
The date of this prospectus is October 6, 2022.
TABLE OF CONTENTS
This prospectus is part of the registration statement that we filed with the Securities and Exchange Commission, or the “SEC,” pursuant to which we may, from time to time, offer and sell or otherwise dispose of the securities covered by this prospectus. As permitted by the rules and regulations of the SEC, the registration statement filed by us includes additional information not contained in this prospectus.
This prospectus and the documents incorporated by reference into this prospectus include important information about us, the securities being offered and other information you should know before investing in our securities. You should not assume that the information contained in this prospectus is accurate on any date subsequent to the date set forth on the front cover of this prospectus or that any information we have incorporated by reference is correct on any date subsequent to the date of the document incorporated by reference, even though this prospectus is delivered or securities are sold or otherwise disposed of on a later date. It is important for you to read and consider all information contained in this prospectus, including the documents incorporated by reference therein, in making your investment decision. You should also read and consider the information in the documents to which we have referred you under “Where You Can Find More Information” and “Incorporation of Certain Information by Reference” in this prospectus.
You should rely only on this prospectus and the information incorporated or deemed to be incorporated by reference in this prospectus. We have not authorized anyone to give any information or to make any representation to you other than those contained or incorporated by reference in this prospectus. If anyone provides you with different or inconsistent information, you should not rely on it. This prospectus does not constitute an offer to sell or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction.
Unless otherwise indicated, information contained or incorporated by reference in this prospectus concerning our industry, including our general expectations and market opportunity, is based on information from our own management estimates and research, as well as from industry and general publications and research, surveys and studies conducted by third parties. Management estimates are derived from publicly available information, our knowledge of our industry and assumptions based on such information and knowledge, which we believe to be reasonable. In addition, assumptions and estimates of our and our industry’s future performance are necessarily uncertain due to a variety of factors, including those described in section of this prospectus titled “Risk Factors.” These and other factors could cause our future performance to differ materially from our assumptions and estimates.
i
We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus is an offer to sell only the securities offered hereby and only under circumstances and in jurisdictions where it is lawful to do so. No dealer, salesperson or other person is authorized to give any information or to represent anything not contained in this prospectus, any applicable prospectus supplement or any free writing prospectuses prepared by or on behalf of us or to which we have referred you or are incorporated by reference. This prospectus is not an offer to sell securities, and it is not soliciting an offer to buy securities, in any jurisdiction where the offer or sale is not permitted.
For investors outside the United States: we have not done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of our securities and the distribution of this prospectus outside the United States.
This prospectus contains summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed, will be filed or will be incorporated by reference as exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of those documents as described in this prospectus under “Where You Can Find More Information.”
This prospectus contains references to trademarks, trade names and service marks belonging to other entities. Solely for convenience, trademarks, trade names and service marks referred to in this prospectus may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that the applicable licensor will not assert, to the fullest extent under applicable law, its rights to these trademarks and trade names. We do not intend our use or display of other entities’ trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, any other entities.
ii
This summary highlights selected information from this prospectus and does not contain all of the information that may be important to you in making an investment decision. This summary is qualified in its entirety by the more detailed information included elsewhere in this prospectus and/or incorporated by reference herein. Before making your investment decision with respect to our securities, you should carefully read this entire prospectus, including the information in our filings with the SEC incorporated by reference into this prospectus.
References in this prospectus to the “Company,” “we,” “us,” “our” and similar words refer to Avenue Therapeutics, Inc.
Our Business
Overview and Product Candidate Development
We are a specialty pharmaceutical company that seeks to develop and commercialize therapies for the treatment of Central Nervous System (“CNS”) diseases. Our current lead product candidate is intravenous (“IV”) Tramadol, for the treatment of post-operative acute pain. Under the terms of certain agreements described herein, we have an exclusive license to develop and commercialize IV Tramadol in the United States.
We have spent significant resources developing IV Tramadol since inception of the company. In 2016, we completed a pharmacokinetic study for IV Tramadol in healthy volunteers as well as an end of Phase 2 meeting with the U.S. Food and Drug Administration (the “FDA”). In the third quarter of 2017, we initiated a Phase 3 development program of IV Tramadol in patients with moderate-to-severe pain following bunionectomy where we announced in May 2018 that the study met its primary endpoint and all key secondary endpoints. In December 2018, we initiated the second Phase 3 trial in patients with moderate-to-severe pain following abdominoplasty upon successful completion of the bunionectomy study. In June 2019, we announced the study met its primary endpoint and all key secondary endpoints. In December 2017, we initiated an open-label safety study, which was completed during the second quarter of 2019. The results showed that IV Tramadol is well-tolerated with a side effect profile consistent with known pharmacology.
In December 2019, we submitted a New Drug Application (“NDA”) for IV Tramadol and received a Complete Response Letter (the “First CRL”) from the FDA in October 2020. In February 2021, we resubmitted the NDA for IV Tramadol. The FDA assigned a Prescription Drug User Fee Act (“PDUFA”) goal date of April 12, 2021 for the resubmitted NDA for IV Tramadol. On June 14, 2021, we announced that we had received a second Complete Response Letter (the “Second CRL”) from the FDA regarding our NDA for IV Tramadol. While efficacy and safety endpoints were met in clinical trials, the FDA expressed a desire for additional safety data related to opioid stacking, which was not directly addressed in the Company’s Phase 3 trials.
We submitted a formal dispute resolution request (“FDRR”) with the Office of Neuroscience of the FDA on July 27, 2021. On August 26, 2021, we received an Appeal Denied Letter from the Office of Neuroscience of the FDA in response to the FDRR submitted on July 27, 2021. On August 31, 2021, we submitted a FDRR with the Office of New Drugs (“OND”) of the FDA. On October 21, 2021, we received a written response from the OND of the FDA stating that the OND needed additional input from an Advisory Committee in order to reach a decision on the FDRR. On February 15, 2022, we had our Advisory Committee meeting with the FDA. In the final part of the public meeting, the Advisory Committee voted yes or no on the following question: “Has the Applicant submitted adequate information to support the position that the benefits of their product outweigh the risks for the management of acute pain severe enough to require an opioid analgesic in an inpatient setting?” The results were 8 ‘yes’ votes and 14 ‘no’ votes. On March 18, 2022, we received an Appeal Denied Letter from the OND in response to the FDRR that also recommend that as a path forward, we “request a meeting with the Division regarding additional studies that can better assess the appropriate clinical setting for the administration of tramadol IV and to evaluate the potential risk for opioid stacking”.
Following the receipt of the Appeal Denied Letter, we submitted a Type A Meeting Request and related briefing document to the FDA on June 17, 2022. The meeting was granted by the Division of Anesthesia, Analgesia, and Addiction Products (“DAAAP”) on June 27, 2022, and scheduled for August 9, 2022. We submitted a briefing document presenting a study design that we believe has the potential to address the concerns around the safety risk of IV Tramadol in combination with other opioid analgesics for the management of moderate-to-moderately-severe pain in adults in a medically supervised healthcare setting that was discussed in detail at the previously disclosed Advisory Committee meeting on February 15, 2022 and in the Appeal Denied letter received on March 18, 2022.
The meeting on August 9, 2022 was a collaborative discussion on the study design and potential path forward. At the meeting, we presented a study design for a single safety clinical trial that we believe could address the concerns regarding risks related to opioid stacking. The FDA stated that the proposed study design appears reasonable and agreed on various study design aspects with the expectation that additional feedback would be provided to us upon review of a more detailed study protocol. We intend to incorporate the FDA’s suggestions from the meeting minutes and submit a detailed study protocol that could form the basis for the submission of a complete response to the second Complete Response Letter for IV Tramadol.
1
We are also anticipating expanding our business with the acquisition of Baergic Bio, Inc. and its asset BAER-101, which would strategically align with Avenue’s goals of building a CNS pipeline. Baergic Bio is a clinical-stage pharmaceutical company founded in December 2019 that focuses on the development of pharmaceutical products for the treatment of CNS disorders. Baergic Bio’s pipeline currently consists of a single compound, BAER-101, a selective GABA-A positive allosteric modulator (“BAER-101”). BAER-101 (formally known as AZD7325) was originally developed by AstraZeneca and has an established safety profile in early clinical trials including over 500 patients. Additional details on the acquisition are described below.
Relationship with Fortress
We were incorporated in Delaware on February 9, 2015, as a wholly owned subsidiary of Fortress Biotech, Inc. (“Fortress”), to develop and market pharmaceutical products for the acute care setting in the United States. Fortress controls a voting majority of our capital stock pursuant to its ownership of a class of preferred stock, some of the features of which have been contractually suspended. We anticipate remaining a majority owned subsidiary of Fortress after the completion of this offering.
Relationship with InvaGen
On November 12, 2018, we, InvaGen Pharmaceuticals Inc. (“InvaGen”) and Madison Pharmaceuticals, Inc. entered into a Stock Purchase and Merger Agreement (the “SPMA”), pursuant to which InvaGen subsequently purchased in 2019, for $35 million, shares of our Common Stock then representing 33.3% of the fully diluted capitalization of our stock. The SPMA also compelled InvaGen to acquire the remaining issued and outstanding shares of our capital stock for approximately $180 million, if certain closing conditions were met on or before April 30, 2021. Such closing conditions were not met as of such date, however, and InvaGen thereafter retained an option to acquire the remaining issued and outstanding shares of our capital stock upon the same terms for so long as the SPMA remained in place. On November 1, 2021, Avenue accrued the contractual right to terminate the SPMA, which it did on the same day in accordance with the agreement’s terms.
Even though we terminated the SPMA on November 1, 2021, InvaGen continues to hold 388,888 shares of our Common Stock and retains certain rights (the “Historic Rights”) pursuant to the Stockholders Agreement, entered into on November 12, 2018 between us, InvaGen and Fortress, including consent rights to certain equity issuances and changes to our capital stock and the right to nominate three members of our board of directors. In connection with, and in anticipation of, this offering, we and InvaGen entered into that certain Share Repurchase Agreement, dated July 28, 2022 (the “Share Repurchase Agreement”). Pursuant to the Share Repurchase Agreement, we agreed to repurchase 100% of the shares in the Company held by InvaGen (the “InvaGen Shares”) for a purchase price of $3 million, conditioned upon the consummation of a financing by the Company. In addition, we agreed to pay InvaGen an additional amount as a contingent fee, payable in the form of seven and a half percent (7.5%) of the proceeds of future financings, up to $4 million. Additionally, in connection with the closing of the repurchase of the InvaGen Shares, all of the Historic Rights will terminate, and the two non-independent members of our board of directors originally selected by InvaGen will resign.
Proposed Acquisition of Baergic Bio
On May 11, 2022, we entered into a stock contribution agreement (the “Contribution Agreement”) with Fortress pursuant to which Fortress agreed to transfer its ownership of a majority of the outstanding shares (common and preferred) in a private subsidiary company of Fortress, Baergic Bio, Inc. (“Baergic Bio”, or “Baergic”), to the Company. Under the Contribution Agreement, Fortress also agreed to assign to the Company certain intercompany agreements existing between Fortress and Baergic, including a Founders Agreement and Management Services Agreement. Consummation of the transactions contemplated by the Contribution Agreement is subject to the satisfaction of certain conditions precedent, including: (i) the closing of an equity financing by the Company resulting in gross proceeds of no less than $7.5 million, (ii) the agreement by InvaGen to (A) have 100% of its shares in the Company repurchased by the Company and (B) terminate certain of the agreements into which it entered with the Company and/or Fortress in connection with InvaGen’s 2019 equity investment in the Company, which will eliminate certain negative consent rights of InvaGen over the Company and restore certain rights and privileges of Fortress in the Company, and (iii) the sustained listing of the Company’s Common Stock on Nasdaq. As previously disclosed, we have since entered into the Share Repurchase Agreement with InvaGen regarding the repurchase of the shares of our Common Stock it holds and the termination of the Historic Rights, although no assurance can be given that the other required consents and approvals for the closing of the Contribution Agreement will be obtained or that the closing conditions will be satisfied in a timely manner or at all.
2
Closing of the acquisition of Baergic under the Contribution Agreement will allow us to take advantage of BAER-101’s unique selectivity profile and further clinical development in areas of unmet need, while affording Baergic Bio with greater access to development expertise and funding. Evaluation and negotiation of the Contribution Agreement was overseen, and execution of the Contribution Agreement was approved, by special committees at the Company and Fortress levels, both of which exclusively comprised of independent and disinterested directors of the respective companies’ boards.
The closing of this offering is expected to satisfy one of the material conditions to the closing of the Baergic Bio contribution, however, there can be no assurance that the other conditions to closing will be satisfied in a timely manner, or at all. You should carefully consider the information set forth under “Risk Factors” in this prospectus.
Even after the closing of this offering, we may need to obtain additional capital through the sale of debt or equity financings or other arrangements to fund our operations and research and development activity; however, there can be no assurance that we will be able to raise needed capital under acceptable terms, if at all. The sale of additional equity may dilute existing stockholders and newly issued shares may contain senior rights and preferences compared to currently outstanding shares of Common Stock. Issued debt securities may contain covenants and limit our ability to pay dividends or make other distributions to stockholders. If we are unable to obtain such additional financing, future operations would need to be scaled back or discontinued.
Summary Risk Factors
Our business is subject to risks of which you should be aware before making an investment decision. The risks described below are a summary of the principal risks associated with an investment in us and are not the only risks we face. You should carefully consider these risk factors, the risk factors described under the heading “Risk Factors”, and the other reports and documents that we have filed with the Securities and Exchange Commission (“SEC”).
Risks Pertaining to the Influence of Fortress
| · | Fortress controls a voting majority of our capital stock pursuant to its ownership of a class of preferred stock, some of the features of which have been contractually suspended (Please see the section titled “Risk Factors – InvaGen retains rights that may prevent us from taking certain actions that could benefit our Company and its stockholders”). |
Risks Pertaining to Our Business
| · | InvaGen retains rights that may prevent us from taking certain actions that could benefit our Company and its stockholders. |
| · | If we fail to satisfy applicable listing standards, our Common Stock may be delisted from the Nasdaq Capital Market, which would impact the liquidity, and potentially the value, of your investment. |
| · | We currently have no drug products for sale, and only one drug product candidate, IV Tramadol. Until the consummation of our acquisition of Baergic Bio, we are dependent on the success of IV Tramadol, and cannot guarantee that we will receive regulatory approval, or that IV Tramadol will be successfully commercialized. |
3
| · | If serious adverse or unacceptable side effects are identified during the development of IV Tramadol or any future product candidates, we may need to abandon or limit our development of some of our product candidates. |
| · | We are an “emerging growth company” and a “smaller reporting company,” and the reduced disclosure requirements applicable to emerging growth companies and smaller reporting companies may make our Common Stock less attractive to investors. |
Risks Pertaining to Our Finances
| · | There is substantial doubt about our ability to continue as a going concern, which may hinder our ability to obtain future financing. |
| · | We have incurred significant losses since our inception. We expect to incur losses for the foreseeable future, and may never achieve or maintain profitability. |
| · | We do not have any products that are approved for commercial sale and therefore do not expect to generate any revenues from product sales in the foreseeable future, if ever. |
| · | Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish proprietary rights. |
Risks Pertaining to Reliance on Third Parties
| · | We rely, and expect to continue to rely, on third parties to conduct our preclinical studies and clinical trials, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials or complying with applicable regulatory requirements. |
| · | We rely on clinical data and results obtained by third parties that could ultimately prove to be inaccurate or unreliable. |
Risks Pertaining to Regulatory Approval Process
| · | We may not receive regulatory approval for IV Tramadol or BAER-101, or our approval may be significantly delayed due to scientific or regulatory reasons. |
| · | We may encounter FDA deficiencies that delay our approval, or we may not obtain approval, if we do not sufficiently address the issues raised by the FDA. |
| · | Even if we respond to the FDA’s requests for information and deficiencies, provide robust scientific justifications and supporting data, there is no guarantee that the FDA will accept our responses, or change its own preliminary conclusions about our product candidate. |
| · | Even if IV Tramadol or BAER-101 receives regulatory approval, which may not occur, it and any other products we may market will remain subject to substantial ongoing regulatory scrutiny. |
| · | We will need to obtain FDA approval of any proposed product brand names, and any failure or delay associated with such approval may adversely impact our business. |
| · | If the Drug Enforcement Agency (“DEA”) decides to reschedule Tramadol from a Schedule IV controlled substance to a more restrictive Schedule IV, Tramadol could lose its competitive advantage, and our related clinical development and regulatory approval could be delayed or prevented. |
4
Risks Pertaining to the Commercialization of Product Candidates
| · | Current and future legislation and regulation may increase the difficulty and cost for us to obtain marketing approval of, and to commercialize, our product candidate and may affect the prices we are able to obtain. |
| · | Public concern regarding the safety of opioid drug products such as IV Tramadol could delay or limit our ability to obtain regulatory approval, result in the inclusion of serious risk information in our labeling, negatively impact market performance, or require us to undertake other activities that may entail additional costs. |
| · | We expect intense competition for IV Tramadol and BAER-101, and new products may emerge that provide different or better therapeutic alternatives for our targeted indications. |
| · | If IV Tramadol or BAER-101 does not achieve broad market acceptance, the revenues that we generate from its sales will be limited. |
Risks Pertaining to Intellectual Property and Potential Disputes Thereof
| · | If we are unable to obtain and maintain patent protection for our technology and products or if the scope of the patent protection obtained is not sufficiently broad, our competitors could develop and commercialize technology and products similar or identical to ours, and our ability to successfully commercialize our technology and products may be impaired. |
| · | If we are sued for infringing intellectual property rights of third parties, it will be costly and time consuming, and an unfavorable outcome in any litigation would harm our business. |
| · | If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed. |
Risks Related to Our Acquisition of Baergic Bio
| · | Our ability to complete the acquisition is dependent on various closing conditions, including the closing of this offering, and consent and approvals from third parties, any of which could adversely affect the acquisition. |
| · | Uncertainty regarding the acquisition can have a negative effect on current operations, as well as future financial and business prospects, including negative impacts on stock prices. |
| · | Substantial expenses will be incurred related to the acquisition of Baergic Bio and the integration of its business operations with our current operation. |
Risks Related to this Offering
| · | If the price of our Common Stock fluctuates significantly, your investment could lose value. |
| · | We will have broad discretion in the use of proceeds of this offering designated for working capital and general corporate purposes. |
| · | The warrants are speculative in nature, holders of the warrants will have no rights as a common stockholder until they acquire shares of our Common Stock and provisions of the warrants could discourage an acquisition of us by a third party. |
Reverse Stock Split
On September 22, 2022, we effected a one-for-fifteen reverse stock split of the shares of our Common Stock by filing on such date the Certificate of Amendment to our Third Amended and Restated Certificate of Incorporation with the Secretary of State of the State of Delaware. No fractional shares were issued as a result of the reverse stock split. Stockholders who would otherwise hold a fractional share of Common Stock received (upon surrender to the exchange agent of certificates representing such shares), a cash payment in lieu thereof, without interest or deduction, rounded to the nearest cent, in an amount equal to the product obtained by multiplying (a) the closing price per share of our common stock as reported on the Nasdaq Stock Market as of September 22, 2022, by (b) the fraction of one share owned by the stockholder.
5
Corporate Information
We are a majority-controlled subsidiary of Fortress. We currently have no subsidiaries, however, we anticipate that after our acquisition of Baergic Bio, that Baergic Bio will become our sole subsidiary.
Avenue Therapeutics, Inc. was incorporated in Delaware on February 9, 2015. Our executive offices are located at 2 Gansevoort Street, 9th Floor, New York, NY 10014. Our telephone number is (781) 652-4500, and our email address is info@avenuetx.com. Information on our website, or any other website, is not incorporated by reference in this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
6
| Units Offered by Us | 2,652,065 units on a "firm commitment" basis, each consisting of one share of Common Stock and one warrant, each warrant exercisable for one share of Common Stock. The shares of Common Stock and warrants that are part of the units are immediately separable and will be issued separately in this offering. The warrants included within the units are exercisable immediately, have an exercise price equal to $3.30 (100% of the public offering price per unit), and expire five years after the date of issuance. |
| Pre-Funded Units Offered by Us: | We are also offering to those purchasers whose purchase of units in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock immediately following the consummation of this offering, 984,300 pre-funded units (each pre-funded unit consisting of one pre-funded warrant to purchase one share of common stock and one warrant to purchase one share of common stock), in lieu of units that would otherwise result in any such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock.
The purchase price of each pre-funded unit will be equal to the price per unit being sold to the public in this offering, minus $0.0001, and the exercise price of each pre-funded warrant included in the pre-funded units will be $0.0001 per share. The pre-funded warrants included in the pre-funded units will be immediately exercisable and may be exercised at any time until all of the pre-funded warrants are exercised in full.
For each pre-funded unit we sell, the number of units we are offering will be decreased on a one-for-one basis. This prospectus also relates to the offering of the shares of common stock issuable upon exercise of the pre-funded warrants. |
| Shares of Common Stock Outstanding Prior to this Offering | 1,475,652 shares of Common Stock |
| Shares of Common Stock Outstanding Following this Offering(1) | 4,127,717 shares of Common Stock |
| Option to Purchase Additional Securities | We have granted the underwriter a 45-day option from the date of this prospectus to purchase up to an aggregate of 545,454 additional shares of Common Stock, additional pre-funded units and/or additional warrants from us in any combination thereof, representing 15% of the securities sold in the offering, solely to cover over-allotments, if any, at the public offering price per share, per pre-funded warrant and per warrant, respectively. |
| Nasdaq Capital Market Ticker Symbol of our Common Stock | ATXI |
7
| Use of proceeds | We estimate that we will receive approximately $10.4 million in net proceeds from this offering (or approximately $12.1 million if the underwriter exercises its over-allotment option in full), after deducting the estimated underwriting discounts and commissions and estimated offering expenses.
We intend to use the net proceeds that we receive from this offering to repurchase all the shares of our Common Stock held by InvaGen for a purchase price of $3.0 million, with the remainder to be used for general corporate purposes and working capital, including the progression of IV Tramadol through regulatory discussions and the development of BAER-101, the product candidate we expect to acquire in connection with our acquisition of Baergic Bio. See “Use of Proceeds” for additional information. |
| Lock-up | We, all of our directors, officers and Fortress Biotech, Inc. have agreed with the underwriter, subject to certain exceptions, not to sell, transfer or dispose of, directly or indirectly, any of our Common Stock or securities convertible into or exercisable or exchangeable for our Common Stock for a period of 12 months, with respect to the Company, and 90 days, with respect to officers, directors and Fortress Biotech, Inc., after the date of the final closing of this offering. See “Underwriting” for more information. |
| Risk factors | Any investment in the Common Stock offered hereby is speculative and involves a high degree of risk. You should carefully consider the information set forth under “Risk Factors” in this prospectus. |
(1) The number of shares of Common Stock to be outstanding after this offering is based on 1,475,652 shares of our Common Stock outstanding as of June 30, 2022, and excludes:
| · | 996 shares of Common Stock issuable upon exercise of outstanding warrants having a weighted-average exercise price of $10.05 per share; |
| · | 21,415 shares of Common Stock issuable upon the vesting and settlement of outstanding restricted stock award/units; |
| · | 122,489 shares of Common Stock reserved for issuance and available for future grant under our 2015 Incentive Plan; |
| · | 545,454 shares of Common Stock issuable upon exercise of the warrants included in the units and the pre-funded units; and | |
| · | 984,300 shares of Common Stock issuable upon exercise of the pre-funded warrants. |
Except as otherwise indicated herein, all information in this prospectus assumes the following
| · | a one-for-fifteen reverse stock split of our Common Stock effective as of September 22, 2022; and |
| · | no exercise by the underwriter of its over-allotment option to purchase additional securities. |
8
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains predictive or “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. All statements other than statements of current or historical fact contained in this prospectus, including statements that express our intentions, plans, objectives, beliefs, expectations, strategies, predictions or any other statements relating to our future activities or other future events or conditions are forward-looking statements. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “will,” “should,” “would” and similar expressions, as they relate to us, are intended to identify forward-looking statements.
These statements are based on current expectations, estimates and projections made by management about our business, our industry and other conditions affecting our financial condition, results of operations or business prospects. These statements are not guarantees of future performance and involve risks, uncertainties and assumptions that are difficult to predict. Therefore, actual outcomes and results may differ materially from what is expressed or forecasted in, or implied by, the forward-looking statements due to numerous risks and uncertainties. Factors that could cause such outcomes and results to differ include, but are not limited to, risks and uncertainties arising from:
| · | expectations for increases or decreases in expenses; |
| · | expectations for the clinical and pre-clinical development, manufacturing, regulatory approval, and commercialization of our pharmaceutical product candidate or any other products we may acquire or in-license; |
| · | our use of clinical research centers and other contractors; |
| · | expectations for incurring capital expenditures to expand our research and development and manufacturing capabilities; |
| · | expectations for generating revenue or becoming profitable on a sustained basis; |
| · | expectations or ability to enter into marketing and other partnership agreements; |
| · | expectations or ability to enter into product acquisition and in-licensing transactions; |
| · | expectations or ability to build our own commercial infrastructure to manufacture, market and sell our product candidate; |
| · | acceptance of our products by doctors, patients or payors; |
| · | our ability to compete against other companies and research institutions; |
| · | our ability to secure adequate protection for our intellectual property; |
| · | our ability to attract and retain key personnel; |
| · | availability of reimbursement for our products; |
| · | estimates of the sufficiency of our existing cash and cash equivalents and investments to finance our operating requirements, including expectations regarding the value and liquidity of our investments; |
| · | the volatility of our stock price; |
| · | expected losses |
9
| · | expectations for future capital requirements; |
| · | uncertainty surrounding the Baergic Bio acquisition; and |
| · | those risks discussed in “Risk Factors” elsewhere in this prospectus, as well as those described in any other filings which we make with the SEC. |
Any forward-looking statements speak only as of the date on which they are made, and we undertake no obligation to publicly update or revise any forward-looking statements to reflect events or circumstances that may arise after the date of this prospectus, except as required by applicable law. Investors should evaluate any statements made by us in light of these important factors.
MARKET AND INDUSTRY DATA AND FORECASTS
We obtained the industry and market data used throughout this prospectus from our own internal estimates and research, as well as from independent market research, industry and general publications and surveys, governmental agencies, publicly available information and research, surveys and studies conducted by third parties. Internal estimates are derived from publicly available information released by industry analysts and third-party sources, our internal research and our industry experience, and are based on assumptions made by us based on such data and our knowledge of our industry and market, which we believe to be reasonable. In some cases, we do not expressly refer to the sources from which this data is derived. In addition, while we believe the industry and market data included in this prospectus is reliable and based on reasonable assumptions, such data involve material risks and other uncertainties and are subject to change based on various factors, including those discussed in the section titled “Risk Factors.” These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties or by us.
10
Our business, results of operations and financial condition and the industry in which we operate are subject to various risks. Accordingly, investing in our securities involves a high degree of risk. We have listed below the most significant risk factors applicable to us, but they do not constitute all of the risks that may be applicable to us. New risks may emerge from time to time, and it is not possible for us to predict all potential risks or to assess the likely impact of all risks. Before making an investment decision, you should carefully consider these risks as well as other information we include or incorporate by reference in this prospectus and any prospectus supplement. This prospectus also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in the forward-looking statements as a result of a number of factors, including the risks described below. See the section titled “Cautionary Note Regarding Forward-Looking Statements.”
Risks Pertaining to Our Stockholders Agreement with InvaGen Pharmaceuticals
InvaGen retains rights that may prevent us from taking certain actions that could benefit our Company and its stockholders.
While the SPMA has been terminated, InvaGen retains certain rights pursuant to the Stockholders Agreement between us and InvaGen. These rights exist as long as InvaGen maintains at least 75% of the shares of Common Stock acquired in the first stage closing. The following are some of the actions that shall not be taken without the prior written consent of InvaGen:
| · | increase in authorized shares of our stock; |
| · | any agreement or transaction that would adversely treat the holders of our shares of Common Stock as compared to the holders of shares of our Class A Preferred Stock; |
| · | issuance of any shares of our capital stock or any securities convertible into, or other rights to acquire, shares of our capital stock (including options, warrants or bonds), except for issuances to our officers for services performed; |
| · | any transfer or license of any asset for less than fair market value, as determined by a recognized independent valuation firm agreed upon by us and InvaGen; or |
| · | entry into any transaction or agreement with any affiliate of ours (including Fortress or its affiliates). |
While we expect that the Stockholders Agreement will terminate in connection with the closing of the Contribution Agreement following this offering under the terms of our redemption agreement with InvaGen, there can be no assurance that the Contribution Agreement or such redemption agreement closes on a timely basis, or at all. Accordingly, for so long as we are bound by the Stockholders Agreement, we may be unable to take any of the above actions, even if we believe doing so would be in the best interests of the Company and/or our stockholders, which could have a material adverse effect on our business, financial condition and results of operations.
Risks Pertaining to the Influence of Fortress
Fortress controls a voting majority of our Common Stock.
Pursuant to the terms of the Class A Preferred Stock held by Fortress, Fortress will be entitled to cast, for each share of Class A Preferred Stock held by Fortress, the number of votes that is equal to 1.1 times a fraction, the numerator of which is the sum of (A) the aggregate number of shares of outstanding Common Stock and (B) the whole shares of Common Stock into which the shares of outstanding the Class A Preferred Stock are convertible and the denominator of which is the aggregate number of shares of outstanding Class A Preferred Stock, or the “Class A Preferred Stock Ratio.” Thus, Fortress will at all times have voting control of us. Further, for a period of ten years from the date of the first issuance of shares of Class A Preferred Stock, the holders of record of the shares of Class A Preferred Stock (or other capital stock or securities issued upon conversion of or in exchange for the Class A Preferred Stock), exclusively and as a separate class, shall be entitled to appoint or elect the majority of our directors.
11
Accordingly, conflicts of interest may arise between Fortress and its affiliates, on the one hand, and us and our other stockholders, on the other hand. In resolving these conflicts of interests, Fortress may favor its own interests and the interests of its affiliates, over the interests of our other stockholders, which could cause a material adverse effect on our business, financial condition and results of operations.
At such time (if ever) as InvaGen no longer holds at least 75% of the shares of our Common Stock it received in its initial 2019 equity subscription, Fortress would have the right to receive a significant grant of shares of our Common Stock annually, which would result in the dilution of your holdings of Common Stock upon each grant, which could reduce their value.
Under the terms of the Amended and Restated Founders Agreement, which became effective September 13, 2016, Fortress is entitled to receive a grant of shares of our Common Stock equal to 2.5% of the gross amount of any equity or debt financing. Additionally, the holders of Class A Preferred Stock, as a class, are to receive an annual dividend, payable in shares of Common Stock in an amount equal to 2.5% of our fully-diluted outstanding capital stock as of the business day immediately prior to the date such dividend is payable. Fortress currently owns all outstanding shares of Class A Preferred Stock. At our Annual Meeting of Stockholders held on June 13, 2018, the Company’s stockholders approved an amendment to the Company’s Third Amended and Restated Certificate of Incorporation, amending the Class A Preferred dividend payment date from February 17 to January 1 of each year. Fortress’ right to receive this dividend was contractually waived in connection with the Waiver and Termination Agreement signed on November 12, 2018 between the Company, Fortress and InvaGen, but Fortress’ right to receive such dividend will be revived at such time (if ever) as InvaGen no longer holds at least 75% of the shares of our Common Stock it received in its initial 2019 equity subscription, which we expect to occur shortly after the closing of this offering. These potential future share issuances to Fortress and any other holder of Class A Preferred Stock will dilute your holdings in our Common Stock and, if our value has not grown proportionately over the prior year, would result in a reduction in the value of your shares. The Amended and Restated Founders Agreement has a term of 15 years and renews automatically for subsequent one-year periods unless terminated by Fortress or upon a Change in Control (as defined in the Amended and Restated Founders Agreement).
We might have received better terms from unaffiliated third parties than the terms we receive in our agreements with Fortress.
We entered into certain agreements with Fortress in connection with our separation from Fortress into an independent company, including the Management Services Agreement, or the “MSA,” and the Founders Agreement, and entered into the Contribution Agreement with Fortress in May 2022. While we believe the terms of these agreements are reasonable, they might not reflect terms that would have resulted from arm’s-length negotiations between unaffiliated third parties. The terms of the agreements relate to, among other things, payment of a royalty on product sales, the provision of employment and transition services and the contribution to us of a majority of the outstanding equity securities of Baergic Bio currently held by Fortress. We might have received better terms from third parties because, among other things, third parties might have competed with each other to win our business. Effective November 12, 2018, the MSA fee and certain payment obligations pursuant to the Founders Agreement were waived under the Waiver and Termination Agreement signed between the Company, Fortress and InvaGen.
12
The ownership by our executive officers and some of our directors of equity securities of Fortress and/or rights to acquire equity securities of Fortress might create, or appear to create, conflicts of interest.
Because of their current or former positions with Fortress, some of our executive officers and directors own shares of Fortress common stock and/or options to purchase shares of Fortress common stock. Their individual holdings of common stock and/or options to purchase common stock of Fortress may be significant compared to their total assets. Ownership by our directors and officers, after our separation from Fortress, of common stock and/or options to purchase common stock of Fortress create or might appear to create conflicts of interest when these directors and officers are faced with decisions that could have different implications for Fortress than for us. For instance, and by way of example, if there were to be a dispute between Fortress and us regarding the calculation of the royalty fee due to Fortress under the terms of the Founders Agreement, then certain of our officers and directors may have and will appear to have a conflict of interest with regard to the outcome of such dispute.
Risks Pertaining to Our Business and Industry
We currently have no drug products for sale, and only one drug product candidate, IV Tramadol. Until the acquisition of Baergic Bio, we are dependent on the success of IV Tramadol and cannot guarantee that this product candidate will receive regulatory approval or be successfully commercialized.
Our business success depends on our ability to obtain regulatory approval to successfully commercialize, market and sell our only product candidate, IV Tramadol, and any significant delays in obtaining approval to commercialize, market and sell IV Tramadol will have a substantial adverse impact on our business and financial condition. Although we expect to become the indirect owner of Baergic Bio’s product candidate, BAER-101, shortly after the consummation of this offering there is no assurance that the acquisition of Baergic Bio contemplated under the Contribution Agreement will occur in a timely manner, or at all. Accordingly, we may remain reliant on IV Tramadol as our sole drug product candidate for the foreseeable future.
If the application for IV Tramadol is approved, our ability to generate revenues from IV Tramadol will depend on our ability to:
| · | establish and maintain agreements with our contract manufacturers, wholesalers, distributors and group purchasing organizations on commercially reasonable terms; |
| · | obtain sufficient quantities of IV Tramadol from qualified third-party manufacturers that manufacture in accordance with Current Good Manufacturing Practices (CGMP) requirements, as required to meet commercial demand at launch and thereafter; |
| · | hire, train, deploy and support our sales force; |
| · | create market demand for IV Tramadol through our own marketing and sales activities, and any other arrangements to promote this product candidate we may later establish; |
| · | conduct such marketing and sales activities in a manner that is compliant with federal and state laws, including restrictions on off-label promotion and anti-kickback requirements; |
| · | obtain and maintain government and private payer reimbursement for our product; and |
| · | maintain patent protection and regulatory exclusivity for IV Tramadol. |
We may not receive regulatory approval for IV Tramadol, BAER-101 (following our acquisition of Baergic Bio) or future product candidates, or its or their approvals may be delayed, which would have a material adverse effect on our business and financial condition.
IV Tramadol, BAER-101 (following our acquisition of Baergic Bio) and other future product candidates and the activities associated with their development and commercialization, including their design, testing, manufacture, safety, efficacy, recordkeeping, labeling, storage, approval, advertising, promotion, sale and distribution, are subject to premarket approval and comprehensive regulation by the FDA, DEA and other regulatory agencies in the United States. Failure to obtain marketing approval for IV Tramadol, BAER-101 or any future product candidates will prevent us from commercializing our product candidates. We have not received approval to market IV Tramadol from regulatory authorities in any jurisdiction. We have only limited experience in conducting preclinical and clinical studies and filing and supporting the applications necessary to gain marketing approvals and expect to rely on third party contract research organizations as well as consultants and vendors to assist us in this process. Securing marketing approval requires the submission of extensive preclinical and clinical data and supporting information to regulatory authorities for each therapeutic indication to establish the product candidate’s safety and efficacy. Securing marketing approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the regulatory authorities.
13
Our product candidates IV Tramadol and BAER-101 (following our acquisition of Baergic Bio), or any future product candidates, must meet FDA’s standards for safety and efficacy, but may be determined not to be effective, to be only moderately effective, to not be safe for use in its intended population, or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude our obtaining marketing approval or prevent or limit commercial use.
In December 2019, we submitted an NDA for IV Tramadol and received the First CRL from the FDA in October 2020. In February 2021, we resubmitted the NDA for IV Tramadol. The FDA assigned a PDUFA goal date of April 12, 2021 for the resubmitted NDA for IV Tramadol. On June 14, 2021, we announced that we had received the Second CRL from the FDA regarding our NDA for IV Tramadol. We submitted a formal dispute resolution request FDRR with the Office of Neuroscience of the FDA on July 27, 2021. On August 26, 2021, we received an Appeal Denied Letter from the Office of Neuroscience of the FDA in response to the FDRR submitted on July 27, 2021. On August 31, 2021, we submitted a FDRR with the Office of New Drugs of the FDA. On October 21, 2021, we received a written response from the Office of New Drugs of the FDA stating that the OND needs additional input from an Advisory Committee in order to reach a decision on the FDRR. On February 15, 2022, we had our Advisory Committee meeting with the FDA. In the final part of the public meeting, the Advisory Committee voted yes or no on the following question: “Has the Applicant submitted adequate information to support the position that the benefits of their product outweigh the risks for the management of acute pain severe enough to require an opioid analgesic in an inpatient setting?” The results were 8 yes votes and 14 no votes. On March 18, 2022, we received an Appeal Denied Letter from the Office of New Drugs in response to the FDRR. We are evaluating next steps with regard to IV Tramadol.
Following the receipt of the Appeal Denied Letter, we submitted a Type A Meeting Request and related briefing document to the FDA on June 17, 2022. The meeting was granted by the Division of Anesthesia, Analgesia, and Addiction Products (“DAAAP”) on June 27, 2022, and scheduled for August 9, 2022. We submitted a briefing document presenting a study design that we believe has the potential to address the concerns around the safety risk of IV Tramadol in combination with other opioid analgesics for the management of moderate-to-moderately-severe pain in adults in a medically supervised healthcare setting that was discussed in detail at the previously disclosed Advisory Committee meeting on February 15, 2022 and in the Appeal Denied letter received on March 18, 2022.
The meeting on August 9, 2022 was a collaborative discussion on the study design and potential path forward. At the meeting, we presented a study design for a single safety clinical trial that we believe could address the concerns regarding risks related to opioid stacking. The FDA stated that the proposed study design appears reasonable and agreed on various study design aspects with the expectation that additional feedback would be provided to us upon review of a more detailed study protocol. We intend to incorporate the FDA’s suggestions from the meeting minutes and submit a detailed study protocol that could form the basis for the submission of a complete response to the second Complete Response Letter for IV Tramadol.
The process of obtaining marketing approvals, both in the United States and abroad, is expensive, may take many years if approval is granted at all, and can vary substantially based upon a variety of factors, including the type, complexity and novelty of the product candidates involved. Changes in marketing approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in the regulatory review process for each submitted product application, may cause delays in the approval or rejection of an application. Regulatory authorities have substantial discretion in the approval process and may refuse to accept any application or may decide that our data is insufficient for approval and require additional preclinical studies or clinical trials. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit or prevent marketing approval of a product candidate. Any marketing approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable.
14
If we experience delays in obtaining approval or if we fail to obtain approval of our product candidate or any future product candidates, the commercial prospects for our product candidates may be harmed and our ability to generate revenue will be materially impaired, thereby negatively impacting our business, financial condition and results of operations.
In addition, even if we were to obtain approval, the approval of the indication for our product candidate by such regulatory authorities may, among other things, be more limited than we request. Such regulatory authorities may not approve the price we intend to charge for our product, may grant approval contingent on the performance of costly post-marketing clinical trials, or may approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate. These regulatory authorities may also require the label to contain warnings, contraindications, or precautions that limit the commercialization of that product. Any of these scenarios could compromise the commercial prospects for our product candidates, including BAER-101 (following our acquisition of Baergic Bio), or any future product candidates.
If serious adverse or unacceptable side effects are identified during the development of our product candidates, we may need to abandon or limit our development of some of our product candidates.
If our product candidate or future product candidates are associated with undesirable side effects in clinical trials or have characteristics that are unexpected, we may need to abandon their development or limit development to more narrow uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective. In our industry, many compounds that initially showed promise in early-stage testing have later been found to cause undesirable side effects that prevented further development of the compound. In the event that our preclinical or clinical trials reveal a high and unacceptable severity and prevalence of side effects, our trials could be delayed, suspended or terminated and the FDA or comparable foreign regulatory authorities could order us to cease further development or deny approval of our product candidate or future product candidates for any or all targeted indications. The FDA could also issue a letter requesting additional data or information prior to making a final decision regarding whether or not to approve a product candidate. The number of requests for additional data or information issued by the FDA in recent years has increased, and resulted in substantial delays in the approval of several new drugs. Undesirable side effects caused by our product candidate or future product candidates could also result in the inclusion of serious risk information in our product labeling, application of burdensome post-market requirements, or the denial of regulatory approval by the FDA or other regulatory authorities for any or all targeted indications, and in turn prevent us from commercializing and generating revenues from the sale of our product candidate. Drug-related side effects could affect patient recruitment or the ability of enrolled patients to complete the trial and could result in potential product liability claims.
For example, some of the adverse events observed in the IV Tramadol clinical trials completed to date include nausea, dizziness, drowsiness, tiredness, sweating, vomiting, dry mouth, somnolence and hypotension. With respect to BAER-101, some of the adverse events observed in clinical trials completed to date include dizziness, somnolence, headache, and euphoric mood.
Additionally, if one or more of our current or future product candidates receives marketing approval, and we or others later identify undesirable side effects caused by this product, a number of potentially significant negative consequences could result, including:
| · | regulatory authorities may require the addition of serious risk-related labeling statements, specific warnings, precautions, or contraindication; |
| · | regulatory authorities may suspend or withdraw their approval of the product, or require the suspension of manufacturing, or the recall of the product from the market; |
15
| · | regulatory authorities may require implementation of burdensome post-market risk mitigation strategies and practices; |
| · | we may be required to change the way the product is administered, conduct additional clinical trials or change the labeling of the product; or |
| · | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining marketing approval and market acceptance of our product candidate or future product candidates or could substantially increase our commercialization costs and expenses, which in turn could delay or prevent us from generating significant revenues from its sale.
We may not be able to manage our business effectively if we are unable to attract and retain key personnel.
We may not be able to attract or retain qualified management and commercial, scientific and clinical personnel in the future due to the intense competition for qualified personnel among biotechnology, pharmaceutical and other businesses. If we are not able to attract and retain necessary personnel to accomplish our business objectives, we may experience constraints that will significantly impede the achievement of our development objectives, our ability to raise additional capital and our ability to implement our business strategy, any of which may have a material adverse effect on our business, financial condition and results of operations.
Our employees, consultants, or third-party partners may engage in misconduct or other improper activities, including those that result in noncompliance with certain regulatory standards and requirements, which could have a material adverse effect on our business.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees, consultants or third-party partners could include intentional failures to comply with FDA regulations, provide accurate information to the FDA, comply with manufacturing standards we have established, comply with federal and state healthcare fraud and abuse laws and regulations, report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee, consultant or third-party misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation, as well as civil and criminal liability. The precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other civil and/or criminal sanctions.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could harm our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. Although we believe that the safety procedures for handling and disposing of these materials comply with the standards prescribed by these laws and regulations, we cannot eliminate the risk of accidental contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties for failure to comply with such laws and regulations.
16
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Our failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
We are an “emerging growth company” and a “smaller reporting company,” and the reduced disclosure requirements applicable to emerging growth companies and smaller reporting companies may make our Common Stock less attractive to investors.
We are an “emerging growth company” as that term is used in the JOBS Act, and may remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the completion of the initial public offering of our Common Stock, (b) in which we have total annual gross revenue of at least $1.07 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our outstanding Common Stock that is held by non-affiliates exceeds $700 million as of the prior June 30, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three year period. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include:
| · | being permitted to provide only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure in our Annual Reports on Form 10-K; |
| · | not being required to comply with the auditor attestation requirements in the assessment of our internal control over financial reporting; |
| · | reduced disclosure obligations regarding executive compensation; and |
| · | exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. |
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of these accounting standards until they would otherwise apply to private companies. We have elected to take advantage of this extended transition period.
We are also a smaller reporting company, and we will remain a smaller reporting company until the fiscal year following the determination that our voting and non-voting common equity held by non-affiliates is more than $250 million measured on the last business day of our second fiscal quarter, or our annual revenues are more than $100 million during the most recently completed fiscal year and our voting and non-voting common equity held by non-affiliates is more than $700 million measured on the last business day of our second fiscal quarter. Similar to emerging growth companies, smaller reporting companies are able to provide simplified executive compensation disclosure, are exempt from the auditor attestation requirements of the Sarbanes-Oxley Act, and have certain other reduced disclosure obligations, including, among other things, being required to provide only two years of audited financial statements and not being required to provide selected financial data, supplemental financial information or risk factors.
We have elected to take advantage of certain of the reduced reporting obligations. We cannot predict whether investors will find our Common Stock less attractive if we rely on these exemptions. If some investors find our Common Stock less attractive as a result, there may be a less active trading market for our Common Stock and our stock price may be reduced or more volatile.
17
We are a “controlled company” within the meaning of Nasdaq listing standards and, as a result, qualify for, and rely on, exemptions from certain corporate governance requirements. You will not have the same protections afforded to stockholders of companies that are subject to such requirements.
We are a “controlled company” within the meaning of Nasdaq listing standards. Under these rules, a company of which more than 50% of the voting power is held by an individual, a group or another company is a “controlled company” and may elect not to comply with certain corporate governance requirements of Nasdaq, including (i) the requirement that a majority of the Board of Directors consist of independent directors, (ii) the requirement that we have a nominating and corporate governance committee that is composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities and (iii) the requirement that we have a compensation committee that is composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities. We have in the past relied on, and intend to continue to rely on, some or all of these exemptions.
Accordingly, you will not have the same protections afforded to stockholders of companies subject to all of the corporate governance requirements of Nasdaq.
Certain of our directors currently serve, and in the past, certain of our officers and directors have served, in similar roles with our parent company, affiliates, related parties and other parties with whom we transact business; ongoing and future relationships and transactions between these parties could result in conflicts of interest.
We sometimes share directors and/or officers with certain of our parent company, affiliates, related parties or other companies with which we transact business, and such arrangements could create conflicts of interest in the future, including with respect to the allocation of corporate opportunities. While we believe that we have put in place policies and procedures to identify such conflicts and that any existing agreements that may give rise to such conflicts and any such policies or procedures were negotiated at arm’s length in conformity with fiduciary duties, such conflicts of interest may nonetheless arise. The existence and consequences of such potential conflicts could expose us to lost profits, claims by our investors and creditors, violations of Nasdaq’s director and audit committee independence rules and harm to our results of operations.
Risks Pertaining to Our Finances
We have incurred significant losses since our inception. We expect to incur losses for the foreseeable future, and may never achieve or maintain profitability.
We are an emerging growth company with a limited operating history. We have focused primarily on in-licensing and developing IV Tramadol, with the goal of supporting regulatory approval for this product candidate. We have incurred losses since our inception in February 2015.
These losses, among other things, have had and will continue to have an adverse effect on our stockholders’ equity and working capital. We expect to continue to incur significant operating losses for the foreseeable future. We also do not anticipate that we will achieve profitability for a period of time after generating material revenues, if ever. If we are unable to generate revenues, we will not become profitable and may be unable to continue operations without continued funding. Because of the numerous risks and uncertainties associated with developing pharmaceutical products, we are unable to predict the timing or amount of increased expenses or when or if, we will be able to achieve profitability. In addition, the Company cannot be certain that additional funding will be available on acceptable terms, or at all.
18
Our net losses may fluctuate significantly from quarter to quarter and year to year. We anticipate that our expenses will increase substantially if:
| · | IV Tramadol, BAER-101 (following our acquisition of Baergic Bio) or other future product candidates are approved for commercial sale, due to the necessity in establishing adequate commercial infrastructure to launch such candidate or candidates without substantial delays, including hiring, sales and marketing personnel, and contracting with third parties for warehousing, distribution, cash collection and related commercial activities; |
| · | we are required by the FDA, or foreign regulatory authorities, to perform studies in addition to those currently expected; |
| · | there are any delays in completing our clinical trials or the development of any of our product candidates; |
| · | we execute other collaborative, licensing or similar arrangements and the timing of payments we may make or receive under these arrangements; |
| · | there are variations in the level of expenses related to our future development programs; |
| · | there are any product liability or intellectual property infringement lawsuits in which we may become involved; and |
| · | there are any regulatory developments affecting IV Tramadol, BAER-101 or the product candidates of our competitors. |
Our ability to become profitable depends upon our ability to generate revenue. To date, we have not generated any revenue from our development stage product, and we do not know when, or if, we will generate any revenue. Our ability to generate revenue depends on a number of factors, including, but not limited to, our ability to:
| · | obtain regulatory approval for IV Tramadol, BAER-101 or any other product candidates that we may license or acquire; |
| · | manufacture commercial quantities of IV Tramadol, BAER-101 or other product candidates, if approved, at acceptable cost levels; and |
| · | develop a commercial organization and the supporting infrastructure required to successfully market and sell our product candidates, if approved. |
Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would depress our value and could impair our ability to raise capital, expand our business, maintain our research and development efforts, diversify our product offerings or even continue our operations. A decline in our value could also cause you to lose all or part of your investment.
Our short operating history makes it difficult to evaluate our business and prospects.
We were incorporated on February 9, 2015, and have only been conducting operations with respect to IV Tramadol since February 17, 2015. We have not yet demonstrated an ability to successfully obtain regulatory approvals, manufacture a commercial scale product, or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful product commercialization. Consequently, any predictions about our future performance may not be as accurate as they could be if we had a history of successfully developing and commercializing pharmaceutical products.
In addition, as a young business, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors. We will need to expand our capabilities to support commercial activities. We may not be successful in adding such capabilities.
19
We expect our financial condition and operating results to continue to fluctuate significantly from quarter to quarter and year to year due to a variety of factors, many of which are beyond our control. Accordingly, you should not rely upon the results of any past quarterly period as an indication of future operating performance.
There is substantial doubt about our ability to continue as a going concern, which may hinder our ability to obtain future financing.
Our audited financial statements as of December 31, 2021 and our unaudited financial statements as of June 30, 2022 have been prepared under the assumption that we will continue as a going concern for the next twelve months. As of December 31, 2021, we had cash and cash equivalents of $3.8 million and an accumulated deficit of $77.0 million. As of June 30, 2022, we had cash and cash equivalents of $0.9 million and an accumulated deficit of $80.5 million. We do not believe that our cash and cash equivalents are sufficient for the next twelve months. As a result of our financial condition and other factors described herein, there is substantial doubt about our ability to continue as a going concern. Our ability to continue as a going concern will depend on our ability to obtain additional funding, as to which no assurances can be given. In addition to this offering, we are continuing to analyze various alternatives, including potentially obtaining lines of credit, debt or equity financings or other arrangements. Our future success depends on our ability to raise capital and/or implement the various strategic alternatives discussed above. We cannot be certain that these initiatives or raising additional capital, whether through selling additional debt or equity securities or obtaining a line of credit or other loan, will be available to us or, if available, will be on terms acceptable to us. If we issue additional securities after the closing of this offering to raise funds, these securities may have rights, preferences, or privileges senior to those of our Common Stock, and our current shareholders may experience dilution. If we are unable to obtain funds when needed or on acceptable terms, we may be required to curtail our current development programs, cut operating costs, forego future development and other opportunities or even terminate our operations.
We will require substantial additional funding, which may not be available to us on acceptable terms, or at all. If we fail to raise the necessary additional capital, we may be unable to raise capital when needed, which would force us to delay, reduce or eliminate our product development programs or commercialization efforts.
Our operations have consumed substantial amounts of cash since inception. We expect to significantly increase our spending to advance the clinical development and potential regulatory approval of our product candidates and launch and commercialize any additional product candidates for which we receive regulatory approval, including building our own commercial organizations to address certain markets. Even after the completion of this offering, we will require additional capital for the further development and potential commercialization our product candidates, as well as to fund our other operating expenses and capital expenditures, and cannot provide any assurance that we will be able to raise funds to complete the development of our product.
We cannot be certain that additional funding will be available on acceptable terms, or at all. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue the development or commercialization of one or more of our product candidates. We may also seek collaborators for product candidates at an earlier stage than otherwise would be desirable or on terms that are less favorable than might otherwise be available. Any of these events could significantly harm our business, financial condition and prospects.
Our future funding requirements will depend on many factors, including, but not limited to:
| · | the potential for delays in our efforts to seek regulatory approval for our product candidate, and any costs associated with such delays; |
| · | the costs of establishing a commercial organization to sell, market and distribute our product candidates; |
| · | the rate of progress and costs of our efforts to prepare for the submission of an NDA for any product candidates that we may in-license or acquire in the future, and the potential that we may need to conduct additional clinical trials to support applications for regulatory approval; |
| · | the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights associated with our product candidates, including any such costs we may be required to expend if our licensors are unwilling or unable to do so; |
20
| · | the cost and timing of securing sufficient supplies of our product candidate from our contract manufacturers in preparation for commercialization; |
| · | the effect of competing technological and market developments; |
| · | the terms and timing of any collaborative, licensing, co-promotion or other arrangements that we may establish; |
| · | if one or more of our product candidates are approved, the potential that we may be required to file a lawsuit to defend our patent rights or regulatory exclusivities from challenges by companies seeking to market generic versions of one or more of our product candidates; and |
| · | the success of the commercialization of one or more of our product candidates. |
In order to carry out our business plan and implement our strategy, we may need to obtain additional financing and may choose to raise additional funds through strategic collaborations, licensing arrangements, public or private equity or debt financing, bank lines of credit, asset sales, government grants, or other arrangements. We cannot be sure that any additional funding, if needed, will be available on terms favorable to us or at all. Furthermore, any additional equity or equity-related financing may be dilutive to our stockholders, and debt or equity financing, if available, may subject us to restrictive covenants and significant interest costs. If we obtain funding through a strategic collaboration or licensing arrangement, we may be required to relinquish our rights to our product candidate or marketing territories.
Our inability to raise capital when needed would harm our business, financial condition and results of operations, and could cause our stock value to decline or require that we wind down our operations altogether.
Raising additional capital may cause dilution to our existing stockholders, including purchasers in this offering, restrict our operations or require us to relinquish proprietary rights.
Until such time, if ever, as we can generate substantial product revenue, we expect to finance our cash needs through a combination of equity offerings, debt financings, grants and license and development agreements in connection with any collaborations. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a stockholder. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends.
If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market any potential product candidates that we would otherwise prefer to develop and market ourselves.
21
If we fail to satisfy applicable listing standards, our common stock may be delisted from the NASDAQ Capital Market.
On September 2, 2021, we received a letter from the Listing Qualifications Department of the Nasdaq Stock Market (“Nasdaq”) notifying us that, based upon its review for the last 30 consecutive business days, we did not meet the continuing listing requirements of Nasdaq Marketplace Rule 5550(b)(2), which requires that we maintain a minimum market value of listed securities of at least $35 million, nor were we in compliance with either of the alternative listing standards, which require maintenance of a minimum of $2.5 million stockholders’ equity or net income of $500,000 from continuing operations in the most recently completed fiscal year, or in two of the three most recently completed fiscal years. Under Nasdaq’s Listing Rules, we had 180 calendar days from the date of the notification to regain compliance, which expired on March 1, 2022. We were unable to regain compliance during this 180-day period. Subsequently, on March 2, 2022, we received an additional notification from the Listing Qualifications Department stating that due to the deficiency, our securities would be delisted from Nasdaq on March 11, 2022, unless we appealed Nasdaq’s determination to a Hearings Panel (the “Panel”). A hearing request would stay the suspension of our securities pending the Panel’s decision. On March 9, 2022, we submitted the hearing request. On April 1, 2022, the Company received a letter from the Office of the General Counsel of The Nasdaq Stock Market LLC which stated that the Nasdaq staff had determined that the Company had regained compliance with the continued listing requirements by having a minimum of $2.5 million in stockholders’ equity and that, consequently, the previously-announced hearing before the Nasdaq Hearings Panel on April 14, 2022, had been cancelled.
On May 24, 2022, the Company received a deficiency letter (the “Nasdaq Letter”) from the Listing Qualifications Department of Nasdaq, notifying the Company that it is not in compliance with Nasdaq Listing Rule 5550(b)(1), which requires the Company to maintain a minimum of $2,500,000 in stockholders’ equity for continued listing on The Nasdaq Capital Market (the “Stockholders’ Equity Requirement”), nor is it in compliance with either of the alternative listing standards, market value of listed securities of at least $35 million or net income of $500,000 from continuing operations in the most recently completed fiscal year, or in two of the three most recently completed fiscal years. The Company’s failure to comply with the Stockholders’ Equity Requirement was based on the Company’s filing of its Quarterly Report on Form 10-Q for the quarter ended March 31, 2022, reporting the stockholders’ equity of $1,159,000.
Pursuant to the Nasdaq Letter, the Company submitted a compliance plan on July 8, 2022. On August 9, 2022, the Company received written notice (the “Notice”) from Nasdaq, stating that Nasdaq had determined that the Company was not in compliance with the rule requiring a minimum bid price of at least $1.00 per share (the “Minimum Bid Price Requirement”) or the Stockholders’ Equity Requirement. The Notice indicated that the Company’s Common Stock would be suspended from trading on Nasdaq unless the Company requested a hearing before the Panel by August 16, 2022. The Company timely requested a hearing with the Panel, which request stayed the trading suspension of the Company’s Common Stock until the completion of the Nasdaq hearing process and the expiration of any additional extension period granted by the Panel following the hearing. The hearing took place on September 22, 2022. On September 29, 2022, the Panel issued a decision granting the Company’s request for continued listing of the Company’s Common Stock through October 31, 2022 to demonstrate compliance with the Stockholders’ Equity Requirement and through October 6, 2022 to satisfy the Minimum Bid Price Requirement.
There can be no assurances, however, that we will be successful in satisfying the Minimum Bid Price Requirement or the Stockholders’ Equity Requirement. Delisting from the NASDAQ could adversely affect our ability to raise additional financing through the public or private sale of equity securities, would significantly affect the ability of investors to trade our securities and would negatively affect the value and liquidity of our Common Stock. Delisting could also have other negative results, including the potential loss of confidence by employees, the loss of institutional investor interest and fewer business development opportunities. If our Common Stock is delisted by the NASDAQ the price of our Common Stock may decline and our Common Stock may be eligible to trade on the OTC Bulletin Board, another over-the-counter quotation system, or on the pink sheets where an investor may find it more difficult to dispose of their Common Stock or obtain accurate quotations as to the market value of our Common Stock. Further, if we are delisted, we would incur additional costs under requirements of state “blue sky” laws in connection with any sales of our securities. These requirements could severely limit the market liquidity of our Common Stock and the ability of our stockholders to sell our Common Stock in the secondary market.
In the event we were to pursue a bankruptcy reorganization under the U.S. Bankruptcy Code, we would be subject to the risks and uncertainties associated with bankruptcy proceedings, including the potential delisting of our Common Stock from trading on Nasdaq.
We continue to experience significant financial and operating challenges that present substantial doubt as to our ability to continue as a going concern. If we continue to experience financial and operating challenges or are unsuccessful or unable to raise additional capital, there is risk that it will be necessary for us to commence reorganization proceedings. In the event we were to pursue such a restructuring, our operations, our ability to develop and execute our business plan and our continuation as a going concern would be subject to the risks and uncertainties associated with bankruptcy proceedings, including, among others: the high costs of bankruptcy proceedings and related fees; our ability to maintain the listing of our Common Stock on the Nasdaq Capital Market; our ability to obtain sufficient financing to allow us to emerge from bankruptcy and execute our business plan post-emergence, and our ability to comply with terms and conditions of that financing; our ability to maintain our relationships with our lenders, counterparties, vendors, suppliers, employees and other third parties; our ability to maintain contracts that are critical to our operations on reasonably acceptable terms and conditions; the ability of third parties to use certain limited safe harbor provisions of the U.S. Bankruptcy Code to terminate contracts without first seeking bankruptcy court approval; and the actions and decisions of third parties who have claims and/or interests in our bankruptcy proceedings that may be inconsistent with our operational and strategic plans. Any reorganization effected under the U.S. Bankruptcy Code will result in a total loss of your investment in our Common Stock.
22
In addition, if we were to commence bankruptcy proceedings, our shares of Common Stock would likely be delisted from trading on Nasdaq. Nasdaq rules provide that securities of a company that trades on Nasdaq may be delisted in the event that such company seeks bankruptcy protection. In response to a Chapter 11 filing, Nasdaq would likely issue a delisting letter immediately following such a filing. If Nasdaq were to issue such a letter, we would have the opportunity to appeal the determination during which time the delisting would be stayed, but if we did not appeal or otherwise were not successful in our appeal, our Common Stock would soon thereafter be delisted and our Common Stock could be traded in the over-the-counter markets. Any delisting of our Common Stock could result in a substantial decline in the value of our Common Stock including, among other reasons, for the reduced liquidity of our Common Stock.
Risks Pertaining to Reliance on Third Parties
If IV Tramadol, BAER-101 (following our acquisition of Baergic Bio), or both products are approved and our contract manufacturer fails to produce the product in the volumes that we require on a timely basis, to produce the product according to the applicable quality standards and requirements, or to comply with stringent regulations applicable to pharmaceutical drug manufacturers, we may face delays in the commercialization of this product candidate, lose potential revenues or be unable to meet market demand.
The manufacture of pharmaceutical products requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls, and the use of specialized processing equipment. We have entered into a development and supply agreement for the completion of pre-commercialization manufacturing development activities and the manufacture of commercial supplies of IV Tramadol. Any termination or disruption of this relationship may materially harm our business and financial condition, and impact any commercialization efforts for this product candidate.
In order to meet anticipated demand for IV Tramadol, if this product candidate is approved, we currently have one manufacturer to provide us clinical and commercial supply of IV Tramadol in accordance with the CGMP requirements. We also may plan to qualify a backup manufacturer, in order to ensure an alternative source and to mitigate any potential supply issues. We have sufficient drug substance for BAER-101 on hand to execute our planned near-term studies, but are in process of identifying future manufacturers.
All of our contract manufacturers must comply with strictly enforced federal, state and, where applicable, foreign regulations, including CGMP requirements enforced by the FDA through its inspectional authority over facilities under the FDCA, as well requirements for controlled substance handling and security requirements enforced by DEA, and while we exercise oversight of our suppliers, we have limited direct control over their compliance with these regulations, as reflected in day-to-day operations. Any failure to comply with applicable regulations may result in fines and civil penalties, suspension of production, suspension or delay in product approval, product seizure or recall, or withdrawal of product approval, and would limit the availability of our product. Any quality or compliance issue, manufacturing defect or error discovered after products have been produced and distributed could result in even more significant consequences, including costly recall procedures, re-stocking costs, damage to our reputation and potential for product liability claims.
If the commercial manufacturers upon whom we rely to manufacture our product candidates we may in-license, fail to deliver sufficient commercial quantities on a timely basis at commercially reasonable prices, we would likely be unable to meet demand for our products and we would lose potential revenues, which could have a material adverse effect on our business, financial condition and results of operations.
23
We rely, and expect to continue to rely, on third parties to conduct our preclinical studies and clinical trials, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials or complying with applicable regulatory requirements.
We have relied on third party contract research organizations and clinical research organizations to conduct some of our preclinical studies and all of our clinical trials for IV Tramadol and may do so for BAER-101 and any other future product candidates. We may continue to rely on third parties, such as contract research organizations, clinical research organizations, clinical data management organizations, medical institutions and clinical investigators, to conduct preclinical studies and clinical trials. The agreements with these third parties might terminate for a variety of reasons, including a failure to perform by the third parties. If we need to enter into alternative arrangements, that could delay our product development activities.
Our reliance on these third parties for research and development activities will reduce our control over these activities but will not relieve us of our legal and regulatory product development responsibilities. For example, we will remain responsible for ensuring that each of our preclinical studies and clinical trials are conducted in accordance with the general investigational plan and protocols for the trial and for ensuring that our preclinical studies are conducted in accordance with good laboratory practice, or “GLP,” as appropriate. Moreover, the FDA requires us to comply with standards, commonly referred to as good clinical practices, or “GCPs,” for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. Regulatory authorities enforce these requirements through periodic inspections of trial sponsors, clinical investigators and trial sites. If we or any of our clinical research organizations fail to comply with applicable GCPs, the clinical data generated in our clinical trials may be deemed unreliable or unacceptable, and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials complies with GCP regulations. In addition, our clinical trials must be conducted using products manufactured and produced in accordance with CGMP regulations. Our failure to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process. We also are required to register ongoing clinical trials and post the results of completed clinical trials on a government-sponsored database, ClinicalTrials.gov, within specified timeframes. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions.
The third parties with whom we have contracted to help perform our preclinical studies or clinical trials may also have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our preclinical studies or clinical trials in accordance with regulatory requirements or our stated protocols, we will not be able to obtain, or may be delayed in obtaining, marketing approvals for our product candidate and will not be able to, or may be delayed in our efforts to, potentially successfully commercialize our product candidate.
If any of our relationships with these third-party contract research organizations or clinical research organizations terminates, we may not be able to enter into arrangements with alternative contract research organizations or clinical research organizations or to do so on commercially reasonable terms. Switching or adding additional contract research organizations or clinical research organizations involves additional cost and requires extensive training and management time and focus. In addition, there is a natural transition period when a new contract research organization or clinical research organization commences work. As a result, delays could occur, which could compromise our ability to meet our desired development timelines. Though we carefully manage our relationships with our contract research organizations or clinical research organizations, there can be no assurance that we will not encounter challenges or delays in the future.
24
We contract with third parties for the manufacture of our product candidates for preclinical and clinical testing and expect to continue to do so for potential commercialization. This reliance on third parties increases the risk that we will not have sufficient quantities of our potential product candidates or products or such quantities at an acceptable cost, which could delay, prevent or impair our development or commercialization efforts.
We do not own any manufacturing facilities or employ any manufacturing personnel. We rely, and expect to continue to rely, on third-party manufacturers to manufacture our product candidates for preclinical and clinical testing, as well as for commercial manufacture, once any of our product candidates receives marketing approval. This reliance on third parties increases the risk that we will not have sufficient quantities of our product candidates or products or such quantities at an acceptable cost or quality, which could delay, prevent or impair our development or potential commercialization efforts.
We may be unable to establish any agreements with such third-party manufacturers or to do so on acceptable terms. Even if we are able to establish agreements with third-party manufacturers, reliance on third-party manufacturers entails additional risks, including, but not necessarily limited to:
| · | reliance on the third party for regulatory compliance and quality assurance; |
| · | raw material or active ingredient shortages from suppliers the third party has qualified for our product; |
| · | the possible breach of the manufacturing agreement by the third party; |
| · | manufacturing delays if our third-party manufacturers give greater priority to the supply of other products over our product candidates or otherwise do not satisfactorily perform according to the terms of the agreement between us; |
| · | the possible misappropriation of our proprietary information, including our trade secrets and know-how; and |
| · | the possible termination or nonrenewal of the agreement by the third party at a time that is costly or inconvenient for us. |
The facilities used by our contract manufacturers to manufacture our product candidate is subject to registration requirements, and inspection by the FDA. A pre-approval inspection may be conducted after the submission of an application to the FDA. Although we will have oversight over our suppliers and manufacturers, we do not directly control the manufacturing operations and processes at these facilities, and therefore rely on, our contract manufacturers to ensure full compliance with CGMP regulations with respect to the day-to-day operations related to the manufacture of our product candidates. Third-party manufacturers may, following an inspection, be subject to a Form FDA-483 or similar inspectional findings, or a Warning Letter, or may not otherwise be able to comply with the CGMP regulations or similar regulatory requirements outside the United States. The failure of our third-party manufacturers to comply with applicable regulations directly impacts our compliance and could result in sanctions being imposed on us, including clinical holds, fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of product candidates or products, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect supplies of our products.
Any products that we may develop may compete with other product candidates and products for access to manufacturing facilities. There may be a limited number of manufacturers that both operate under CGMP regulations and are capable of manufacturing for us. Any performance failure on the part of our existing or future manufacturers could delay clinical development or marketing approval. We do not currently have arrangements in place for redundant supply or a second source for bulk drug substance. If our current contract manufacturers cannot perform as agreed, we may be required to replace such manufacturers. We may incur added costs and delays in identifying and qualifying any replacement manufacturers.
The DEA restricts the importation of a controlled substance finished drug product when the same substance is commercially available in the United States, which could reduce the number of potential alternative manufacturers for IV Tramadol.
Our current and anticipated future dependence upon others for the manufacture of our product candidate may adversely affect our future profit margins and our ability to potentially commercialize any products that receive marketing approval on a timely and competitive basis.
25
We also expect to rely on other third parties to store and distribute drug supplies for our clinical trials. Any performance failure on the part of our distributors could delay clinical development or marketing approval of our product candidates or potential commercialization of our products, producing additional losses and depriving us of potential product revenue.
We rely on clinical data and results obtained by third parties that could ultimately prove to be inaccurate or unreliable.
As part of our strategy to mitigate development risk, we sought to develop a product candidate with a validated mechanism of action, and we utilize biomarkers to assess potential clinical efficacy early in the development process. This strategy necessarily relies upon clinical data and other results obtained by third parties that may ultimately prove to be inaccurate or unreliable. Further, such clinical data and results may be based on products or product candidates that are significantly different from our product candidate or future product candidates. If the third-party data and results we rely upon prove to be inaccurate, unreliable or not applicable to our product candidate or future product candidate, we could make inaccurate assumptions and conclusions about our product candidates and our research and development efforts could be compromised and called into question during the review or any marketing applications we submit.
Risks Pertaining to Regulatory Approval Process
We may not receive regulatory approval for IV Tramadol, or our approval may be significantly delayed due to scientific or regulatory reasons.
While we expect to acquire BAER-101 in connection with our acquisition of Baergic Bio shortly after the closing of this offering, we continue to pursue regulatory approval for IV Tramadol, our only current drug candidate. However, in light of recently disclosed developments, there is considerable doubt about our ability to obtain regulatory approval for IV Tramadol. In December 2019, we submitted an NDA for IV Tramadol and received the First CRL from the FDA in October 2020. In February 2021, we resubmitted the NDA for IV Tramadol. The FDA assigned a PDUFA goal date of April 12, 2021 for the resubmitted NDA for IV Tramadol. On June 14, 2021, we announced that we had received the Second CRL from the FDA regarding our NDA for IV Tramadol. We submitted a formal dispute resolution request FDRR with the Office of Neuroscience of the FDA on July 27, 2021. On August 26, 2021, we received an Appeal Denied Letter from the Office of Neuroscience of the FDA in response to the FDRR submitted on July 27, 2021. On August 31, 2021, we submitted a FDRR with the Office of New Drugs of the FDA. On October 21, 2021, we received a written response from the Office of New Drugs of the FDA stating that the OND needs additional input from an Advisory Committee in order to reach a decision on the FDRR. On February 15, 2022, we had our Advisory Committee meeting with the FDA. In the final part of the public meeting, the Advisory Committee voted yes or no on the following question: “Has the Applicant submitted adequate information to support the position that the benefits of their product outweigh the risks for the management of acute pain severe enough to require an opioid analgesic in an inpatient setting?” The results were 8 yes votes and 14 no votes. On March 18, 2022, we received an Appeal Denied Letter from the Office of New Drugs in response to the FDRR.
Following the receipt of the Appeal Denied Letter, we submitted a Type A Meeting Request and related briefing document to the FDA on June 17, 2022. The meeting was granted by the Division of Anesthesia, Analgesia, and Addiction Products (“DAAAP”) on June 27, 2022, and scheduled for August 9, 2022. We submitted a briefing document presenting a study design that we believe has the potential to address the concerns around the safety risk of IV Tramadol in combination with other opioid analgesics for the management of moderate-to-moderately-severe pain in adults in a medically supervised healthcare setting that was discussed in detail at the previously disclosed Advisory Committee meeting on February 15, 2022 and in the Appeal Denied letter received on March 18, 2022.
The meeting on August 9, 2022 was a collaborative discussion on the study design and potential path forward. At the meeting, we presented a study design for a single safety clinical trial that we believe could address the concerns regarding risks related to opioid stacking. The FDA stated that the proposed study design appears reasonable and agreed on various study design aspects with the expectation that additional feedback would be provided to us upon review of a more detailed study protocol. We intend to incorporate the FDA’s suggestions from the meeting minutes and submit a detailed study protocol that could form the basis for the submission of a complete response to the second Complete Response Letter for IV Tramadol.
26
Even if IV Tramadol receives regulatory approval, which may not occur, it and any other products we may market will remain subject to substantial regulatory scrutiny.
IV Tramadol and any other product candidates we may license or acquire will also be subject to ongoing regulatory and compliance requirements, including regular inspections by the FDA and other regulatory authorities. These requirements relate to, among others, labeling, packaging, storage, advertising, promotion, record-keeping and submission of safety and other post-market information and reports, registration and listing requirements, ongoing CGMP requirements relating to manufacturing, quality control, quality assurance and corresponding maintenance of records and documents, requirements regarding the distribution of samples to physicians and recordkeeping of the drug.
The FDA may also impose requirements for costly post-marketing studies or clinical trials and surveillance programs to monitor the safety or efficacy of the product. The FDA closely regulates the post-approval marketing and promotion of drugs to ensure drugs are marketed only for the approved indications and in accordance with the approved labeling. The FDA imposes stringent restrictions on manufacturers’ communications regarding off-label use and off-label information and if we do not market our products for only their approved indications and on-label information, we may be subject to enforcement action for off-label marketing as well as false claims liability. Violations of the FDCA relating to the promotion of prescription drugs may lead to investigations alleging violations of federal and state health care fraud and abuse laws, as well as state consumer protection laws.
In addition, later discovery of previously unknown adverse events or other problems with our product, manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may yield various results, including:
| · | restrictions on such product, operations, manufacturers or manufacturing processes; |
| · | restrictions or new requirements related to the promotion, labeling or marketing of a product; |
| · | restrictions on product distribution or use, including import and export restrictions; |
| · | requirements to conduct post-marketing studies or clinical trials; |
| · | Form FDA-483 findings, or warning letters; |
| · | recall of the product, or withdrawal of the product from the market; |
| · | refusal to approve pending applications or supplements to approved applications that we submit; |
| · | fines, restitution or disgorgement of profits; |
| · | suspension or withdrawal of marketing or regulatory approvals; |
| · | suspension of any ongoing clinical trials; |
| · | refusal to permit the import or export of our product; |
| · | product seizure; or |
| · | injunctions or the imposition of civil or criminal penalties. |
The FDA’s policies, as well as policies of the DEA, who has jurisdiction over controlled substances and opioids, may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidate. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained.
27
We will need to obtain FDA approval of any proposed product brand names, and any failure or delay associated with such approval may adversely impact our business.
A pharmaceutical product candidate cannot be marketed in the United States or many other countries until we have completed a rigorous and extensive regulatory review processes, including obtaining the approval of a brand name. Any brand names we intend to use for our product candidates will require approval from the FDA regardless of whether we have secured a formal trademark registration from the U.S. Patent and Trademark Office, or “USPTO.” The FDA typically conducts a review of proposed product brand names, including an evaluation of potential for confusion with other product names. The FDA may also object to a product brand name if it believes the name inappropriately implies medical claims. If the FDA objects to any of our proposed product brand name, we may be required to adopt an alternative brand name for our product candidate. If we adopt an alternative brand name, we would lose the benefit of our existing trademark applications for such product candidate and may be required to expend significant additional resources in an effort to identify a suitable product brand name that would qualify under applicable trademark laws, not infringe the existing rights of third parties and be acceptable to the FDA. We may be unable to build a successful brand identity for a new trademark in a timely manner or at all, which would limit our ability to potentially commercialize our product candidate.
Our current and future relationships with customers and third-party payors in the United States and elsewhere may be subject, directly or indirectly, to applicable anti-kickback, fraud and abuse, false claims, transparency, health information privacy and security and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm, administrative burdens and diminished profits and future earnings.
Healthcare providers, physicians and third-party payors in the United States and elsewhere will play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our future arrangements with third-party payors, distributors, retailers, marketers and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations, including, without limitation, the federal Anti-Kickback Statute, the federal False Claims Act, and similar state or foreign laws which may constrain the business or financial arrangements and relationships through which we sell, market and distribute any product candidates for which we obtain marketing approval. In addition, we may be subject to transparency laws and patient privacy regulation by U.S. federal and state governments and by governments in foreign jurisdictions in which we conduct our business. The applicable federal, state and foreign healthcare laws and regulations that may affect our ability to operate include, but are not necessarily limited to:
| · | the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal and state healthcare programs, such as Medicare and Medicaid; |
| · | federal civil and criminal false claims laws and civil monetary penalty laws, including the federal False Claims Act, which impose criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, including the Medicare and Medicaid programs, claims for payment that are false or fraudulent, making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government, or the knowing retention of an overpayment from government health care programs; the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
| · | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, which impose obligations on covered healthcare providers, health plans, and healthcare clearinghouses, as well as their business associates that create, receive, maintain or transmit individually identifiable health information for or on behalf of a covered entity, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
28
| · | the federal Open Payments program, which requires manufacturers of certain drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to report annually to the Centers for Medicare & Medicaid Services, or “CMS,” information related to “payments or other transfers of value” made to physicians, which is defined to include doctors, dentists, optometrists, podiatrists and chiropractors, and certain teaching hospitals and applicable manufacturers to report annually to CMS ownership and investment interests held by the physicians and their immediate family members; and |
| · | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third party payors, including private insurers; state and foreign laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers; state and foreign laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state and foreign laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations may involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, including, without limitation, damages, fines, imprisonment, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations, which could have a material adverse effect on our business. If any of the physicians or other healthcare providers or entities with whom we expect to do business, including our collaborators, is found not to be in compliance with applicable laws, it may be subject to criminal, civil or administrative sanctions, including exclusions from participation in government healthcare programs, which could also materially affect our business, financial condition and results of operations..
Regulatory approval for any approved product is limited by the FDA to those specific indications and conditions for which clinical safety and efficacy have been demonstrated.
Any regulatory approval is limited to the specific labeled indication(s) for which a product is deemed to be safe and effective by the FDA. In addition to the FDA approval required for new formulations, any new indication for an approved product also requires FDA approval. If we are not able to obtain FDA approval for any desired future indications for our product, our ability to effectively potentially market and sell our product may be reduced and our business may be adversely affected.
While physicians may choose to prescribe drugs for uses that are not described in the product’s approved labeled indication, or for uses that differ from those tested in clinical studies, and thus the basis for approval by the regulatory authorities, our ability to promote the products is limited to those indications that are specifically approved by the FDA. These “off-label” uses are common across medical specialties and may constitute an appropriate treatment for some patients in varied circumstances. Regulatory authorities in the United States generally do not regulate the practice of medicine by physicians with respect to their choice of treatments. Regulatory authorities do, however, restrict communications by pharmaceutical companies in terms of their ability to promote off-label uses or disseminate off-label information. If our promotional activities fail to comply with these requirements, we may be subject to regulatory, compliance, or enforcement action by, these authorities. In addition, our failure to follow FDA requirements relating to promotion and advertising may result in a Warning Letter, cause the FDA to suspend or withdraw an approved product from the market, require a recall, require the issuance of corrective advertising, institute fines, or could result in disgorgement of money, operating restrictions, injunctions or civil or criminal prosecution by the government, any of which could harm our reputation and business.
29
If the DEA decides to reschedule Tramadol from a Schedule IV controlled substance to a more restrictive Schedule, IV Tramadol could lose its competitive advantage, and our related clinical development and regulatory approval could be delayed or prevented.
In July 2014, the DEA classified Tramadol as a Schedule IV controlled substance. In comparison, other opioids, which have a high potential for abuse, are classified as Schedule I and II controlled substances. If approved, IV Tramadol will be the only intravenous Schedule IV opioid on the market. However, in the current environment where the opioid epidemic is a recognized problem in the United States, there is a possibility that the DEA could reschedule Tramadol to a more restrictive classification (Schedule I, II or III). Such a rescheduling, or other similar action by DEA, would severely impair IV Tramadol’s current competitive advantage over traditional opioids and may affect our ability to potentially market IV Tramadol as a safe alternative pain management product.
Risks Pertaining to the Commercialization of Product Candidates
Current and future legislation and regulation may increase the difficulty and cost for us to obtain marketing approval of, and to commercialize, our product candidate and may affect the prices we are able to obtain.
In the United States, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidate, restrict or regulate post-approval activities, and affect our ability to profitably sell any product candidates for which we obtain marketing approval.
In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or the MMA, changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and certain disabled people and introduced a reimbursement methodology based on average sales prices for physician-administered drugs. In addition, this law provided authority for limiting the number of drugs that will be covered in any therapeutic class. Cost reduction initiatives and other provisions of this law and future laws could decrease the coverage and price that we will receive for any approved products. While the MMA only applies to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own payment rates. Therefore, any limitations in reimbursement that results from the MMA may result in reductions in payments from private payors.
In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, collectively the “ACA,” became law. The ACA is a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms.
Among the provisions of the ACA of importance to our potential product candidate are the following:
| · | an annual, nondeductible fee on any entity that manufactures or imports specified branded prescription drugs and biological products; |
| · | an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program; |
| · | expansion of healthcare fraud and abuse laws, including the False Claims Act and the Anti-Kickback Statute, new government investigative powers, and enhanced penalties for noncompliance; |
| · | new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices; |
| · | extension of manufacturers’ Medicaid rebate liability to drugs dispensed to Medicaid managed care organization enrollees; |
| · | expansion of eligibility criteria for Medicaid programs; |
| · | expansion of the entities eligible for discounts under 340B Drug Pricing Program; |
| · | new requirements to report financial arrangements with physicians and teaching hospitals; |
| · | a new requirement to annually report drug samples that manufacturers and distributors provide to physicians; and |
| · | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research. |
30
At the end of 2017, Congress passed the Tax Cuts and Jobs Act, which repealed the penalty for individuals who fail to maintain minimum essential health coverage as required by the ACA. Following this legislation, Texas and 19 other states filed a lawsuit alleging that the ACA is unconstitutional as the individual mandate was repealed, undermining the legal basis for the Supreme Court’s prior decision. On December 14, 2018, Texas Federal District Court Judge Reed O’Connor issued a ruling declaring that the ACA in its entirety is unconstitutional. Upon appeal, the Fifth Circuit upheld the district court’s ruling that the individual mandate is unconstitutional. However, the Fifth Circuit remanded the case back to the district court to conduct a more thorough assessment of the constitutionality of the entire ACA despite the individual mandate being unconstitutional. The Supreme Court agreed to hear the case on appeal from the Fifth Circuit on March 2, 2020 and held oral arguments on November 10, 2020. While this lawsuit has no immediate legal effect on the ACA and its provisions, it is ongoing and the outcome may have a significant impact on our business.
The Bipartisan Budget Act of 2018, the “BBA,” which set government spending levels for Fiscal Years 2018 and 2019, revised certain provisions of the ACA. Specifically, beginning in 2019, the BBA increased manufacturer point-of-sale discounts off negotiated prices of applicable brand drugs in the Medicare Part D coverage gap from 50% to 70%, ultimately increasing the liability for brand drug manufacturers. Further, this mandatory manufacturer discount applied to biosimilars beginning in 2019.
There have been, and likely will continue to be, legislative and regulatory proposals at the federal and state levels directed at broadening the availability of healthcare and containing or lowering the cost of healthcare products and services. We cannot predict the initiatives that may be adopted in the future. The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce costs of healthcare may adversely affect:
| · | the demand for any products for which we may obtain regulatory approval; |
| · | our ability to set a price that we believe is fair for our products; |
| · | our ability to generate revenues and achieve or maintain profitability; |
| · | the level of taxes that we are required to pay; and |
| · | the availability of capital. |
In addition, governments may impose price controls, which may adversely affect our future profitability. In January 2020, President Trump signed into law the U.S.-Mexico-Canada (USMCA) trade deal into law. As enacted, there are no commitments with respect to biological product intellectual property rights or data protection, which may create an unfavorable environment across these three countries
We expect that the ACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and additional downward pressure on the payment that we receive for any approved drug. Any reduction in reimbursement from Medicare or other government healthcare programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to potentially generate revenue, attain profitability, or commercialize our product.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals, if any, of our product candidate, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing conditions and other requirements.
31
Public concern regarding the safety of opioid drug products such as IV Tramadol could delay or limit our ability to obtain regulatory approval for this product, result in the inclusion of serious risk information in our labeling, negatively impact market performance, or require us to undertake other activities that may entail additional costs.
In light of widely publicized events concerning the safety risk of certain drug products, the FDA, members of Congress, the Government Accountability Office, medical professionals and the general public have raised concerns about potential controlled substance drug safety issues. These events have resulted in the withdrawal of drug products, revisions to drug labeling that further limit use of the drug products and the establishment of risk management programs. The Food and Drug Administration Amendments Act of 2007, or “FDAAA,” grants significant expanded authority to the FDA much of which is aimed at improving the safety of drug products before and after approval. In particular, the new law authorizes the FDA to, among other things, require post-approval studies and clinical trials, mandate changes to drug labeling to reflect new safety information and require risk evaluation and mitigation strategies for certain drugs, including certain currently approved drugs. It also significantly expands the federal government’s clinical trial registry and results databank, which we expect will result in significantly increased government oversight of clinical trials. Under the FDAAA, companies that violate these and other provisions of the new law are subject to substantial civil monetary penalties, among other regulatory, civil and criminal penalties. The increased attention to drug safety issues may result in a more cautious approach by the FDA in its review of data from our clinical trials. Data from clinical trials may receive greater scrutiny, particularly with respect to safety, which may make the FDA or other regulatory authorities more likely to require additional preclinical studies or clinical trials. If the FDA requires us to conduct additional preclinical studies or clinical trials prior to approving IV Tramadol, our ability to obtain approval of this product candidate will be delayed. If the FDA requires us to provide additional clinical or preclinical data following the approval of IV Tramadol, the indications for which this product candidate is approved may be limited or there may be specific warnings or limitations on production dosing, and our efforts to commercialize IV Tramadol may be otherwise adversely impacted.
Rising public, medical, Congressional, and agency concern around the prescription of controlled substance drug products to patients and a growing movement to reduce the use of opioid drug products, to develop abuse-deterrent products, and to prevent dependence also could negatively impact our ability to commercialize and generate revenue from IV Tramadol if it is approved for marketing in the United States. Congress has enacted several laws intended to address opioid use disorder, including the Comprehensive Addiction and Recovery Act (CARA) in 2016, the 21st Century Cures Act (Cures Act) in 2016, and the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act (the SUPPORT Act) in 2018. These laws primarily focus on funding for treatment, research, and education, but also include provisions intended to encourage reduction in opioid use, such as funding for research on non-opioid pain treatments. Other legislative and administrative measures at the state and federal level include, or may include in the future, restrictions and limitations on opioid prescribing, limitations on opioid doses dispensed per episode of care, labeling requirements specific to opioids, limitations on FDA approval of opioids, assessment of fees against opioid manufacturers, or reimbursement disincentives specific to opioids.
We expect intense competition for IV Tramadol and BAER-101, and new products may emerge that provide different or better therapeutic alternatives for our targeted indications.
The biotechnology and pharmaceutical industries are subject to rapid and intense technological change. We face, and will continue to face, competition in the development and marketing of IV Tramadol from academic institutions, government agencies, research institutions and biotechnology and pharmaceutical companies and expect to face similar competition for BAER-101. There can be no assurance that developments by others will not render IV Tramadol or BAER-101 obsolete or noncompetitive. Furthermore, new developments, including the development of other drug technologies and methods of preventing the incidence of disease, occur in the pharmaceutical industry at a rapid pace. These developments may render IV Tramadol or BAER-101 obsolete or noncompetitive.
32
IV Tramadol will compete with well-established products with similar indications. Competing products available for the management of pain include Ofirmev (IV acetaminophen) and IV formulations of NSAIDs such as Dyloject (diclofenac), Toradol (ketorolac), Anjeso (meloxicam) and Caldolor (ibuprofen). In addition, we also expect to compete with agents such as Exparel, a liposome injection of bupivacaine indicated for administration into the surgical site to produce postsurgical analgesia. In addition to approved products, there are a number of product candidates in development for the management of acute pain. The late-stage pain development pipeline is replete with reformulations and fixed-dose combination products of already available therapies. Among specific drug classes, opioid analgesics and NSAIDs represent the greatest number of agents in development. Most investigational opioids that have reached the later stages of clinical development are new formulations of already marketed opioids. Likewise, investigational NSAIDs — mostly lower dose injectable reformulations of already approved compounds — are another significant area of late-stage drug development in the postoperative pain space. Competitors may seek to develop alternative formulations of IV centrally acting synthetic opioid analgesics for our targeted indications that do not directly infringe on our in-licensed patent rights.
Competitors in the GABA-A space are in the clinic and include Cerevel Therapeutics (Darigabat), RespireRx Pharmaceuticals (KRM-II-81), Saniona AB (SAN711), and Engrail Therapeutics (ENX101). The commercial opportunity for IV Tramadol and BAER-101 could be significantly harmed if competitors are able to develop alternative formulations outside the scope of our in-licensed patents. Compared to us, many of our potential competitors have substantially greater:
| · | capital resources; |
| · | development resources, including personnel and technology; |
| · | clinical trial experience; |
| · | regulatory experience; |
| · | expertise in prosecution of intellectual property rights; and |
| · | manufacturing, distribution and sales and marketing experience. |
As a result of these factors, our competitors may obtain regulatory approval of their products more rapidly than we are able to or may obtain patent protection or other intellectual property rights that limit our ability to develop or potentially commercialize IV Tramadol or BAER-101 (following our acquisition of Baergic Bio). Our competitors may also develop drugs that are more effective, safe, useful and less costly than ours and may be more successful than us in manufacturing and marketing their products.
If the government or third-party payors fail to provide adequate coverage and payment rates for IV Tramadol, BAER-101 or any future products we may license or acquire in the future, if any, or if hospitals choose to use therapies that are less expensive, our potential revenue and prospects for profitability will be limited.
Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower-cost drugs and may be incorporated into existing payments for other services. In both domestic and foreign markets, our sales of any future products will depend in part upon the availability of coverage and reimbursement from third party payors. Such third-party payors include government health programs such as Medicare and Medicaid, managed care providers, private health insurers and other organizations. In particular, many U.S. hospitals receive a fixed reimbursement amount per procedure for certain surgeries and other treatment therapies they perform. Because this amount may not be based on the actual expenses the hospital incurs, hospitals may choose to use therapies which are less expensive when compared to our product candidate or future product candidates. Accordingly, IV Tramadol, BAER-101 (following our acquisition of Baergic Bio) or any other product candidates that we may in-license or acquire, if approved, will face competition from other therapies and drugs for these limited hospital financial resources. We may need to conduct post-marketing studies in order to demonstrate the cost-effectiveness of any future products to the satisfaction of hospitals, other target customers and their third-party payors. Such studies might require us to commit a significant amount of management time and financial and other resources. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by implementation of recently promulgated regulations that permit importation of drugs from countries where they may be sold at lower prices than in the United States. Our future product might not ultimately be considered cost-effective. Adequate third-party coverage and reimbursement might not be available to enable us to maintain price levels sufficient to realize an appropriate return on investment in product development.
33
If none of our product candidates achieves broad market acceptance, the potential revenues that we generate from sales will be limited.
The commercial success of IV Tramadol, BAER-101 (following our acquisition of Baergic Bio), or both, if approved, will depend upon its acceptance by the medical community, the ability to ensure that the drug is included in hospital formularies, and coverage and reimbursement for the drug by third party payors, including government payors. The degree of market acceptance of IV Tramadol, BAER-101 or any other product candidate we may license or acquire would depend on a number of factors, including, but not necessarily limited to:
| · | the efficacy and safety as demonstrated in clinical trials; |
| · | the safety and use of IV Tramadol or BAER-101 in its intended patient population; |
| · | the timing of market introduction of such product candidate as well as competitive products; |
| · | the clinical indications for which the drug is approved; |
| · | acceptance by physicians, major operators of cancer clinics and patients of the drug as a safe and effective treatment; |
| · | the safety of such product candidate seen in a broader patient group (i.e., real world use); |
| · | the availability, cost and potential advantages of alternative treatments, including less expensive generic drugs; |
| · | the availability of adequate reimbursement and pricing by third party payors and government authorities; |
| · | the relative convenience and ease of administration of the product candidate for clinical practices; |
| · | the product labeling or product insert required by the FDA or regulatory authority in other countries, including any contradictions, warnings, drug interactions, or other precautions; |
| · | the approval, availability, market acceptance and reimbursement for a companion diagnostic, if any; |
| · | the prevalence and severity of adverse side effects; |
| · | the effectiveness of our sales and marketing efforts; |
| · | changes in the standard of care for the targeted indications for our product candidate or future product candidates, which could reduce the marketing impact of any superiority claims that we could make following FDA approval; and |
| · | potential advantages over, and availability of, alternative treatments. |
34
If any product candidate that we develop does not provide a treatment regimen that is as beneficial as, or is not perceived as being as beneficial as, the current standard of care or otherwise does not provide patient benefit, that product candidate, if approved for commercial sale by the FDA or other regulatory authorities, likely will not achieve market acceptance. Our ability to effectively promote and potentially sell IV Tramadol, BAER-101 (following our acquisition of Baergic Bio) and any other product candidates we may license or acquire in the hospital marketplace will also depend on pricing and cost effectiveness, including our ability to produce a product at a competitive price and achieve acceptance of the product onto hospital formularies, as well as our ability to obtain sufficient third-party coverage or reimbursement. Since many hospitals are members of group purchasing organizations, which leverage the purchasing power of a group of entities to obtain discounts based on the collective buying power of the group, our ability to potentially attract customers in the hospital marketplace will also depend on our ability to effectively potentially promote our product candidate to group purchasing organizations. We will also need to demonstrate acceptable evidence of safety and efficacy, as well as relative convenience and ease of administration. Market acceptance could be further limited depending on the prevalence and severity of any expected or unexpected adverse side effects associated with our product candidate. If our product candidate is approved but does not achieve an adequate level of acceptance by physicians, health care payors and patients, we may not potentially generate sufficient revenue from this product, and we may not become or remain profitable. In addition, our efforts to educate the medical community and third-party payors on the benefits of our product candidate may require significant resources and may never be successful.
If we are unable to establish sales, and marketing capabilities or to enter into agreements with third parties to market and sell our product candidate, we may not be successful in commercializing our product candidates if and when they are approved.
We currently do not have a marketing or sales organization for the marketing and sales of pharmaceutical products since we currently have no drug products for sale, and only one drug product candidate, IV Tramadol. In order to potentially commercialize any product candidate that receives marketing approval, we would need to build out marketing, sales, managerial and other non-technical capabilities or enter into agreements with third party contract organizations to perform these services, and we may not be successful in doing so. In the event of successful development and regulatory approval of IV Tramadol, BAER-101 or another product candidate, we might have to build a targeted specialist sales force to market or co-promote the product. There are risks involved with establishing our own sales and marketing capabilities. For example, recruiting and training a sales force is expensive and time consuming and could delay any product launch. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel.
Factors that may inhibit our potential efforts to successfully commercialize our future product, if any, using our own sales and marketing capabilities include, but are not necessarily limited to:
| · | our inability to recruit, train and retain adequate numbers of effective sales and marketing personnel; |
| · | the inability of sales personnel to obtain access to physicians or persuade adequate numbers of physicians to prescribe any future products; |
| · | the lack of complementary or other products to be offered by sales personnel, which may put us at a competitive disadvantage from the perspective of sales efficiency relative to companies with more extensive product lines; and |
| · | unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
As an alternative to establishing our own sales force, we may choose to partner with third parties that have well-established direct sales forces to sell, market and distribute our products. There are risks involved with partnering with third party sales forces, including ensuring adequate training on the product, regulatory, and compliance requirements associated with promotion of the product.
35
If we breach the agreement under which we license rights to IV Tramadol, we could lose the ability to continue to develop and potentially commercialize this product candidate.
In February 2015, Fortress obtained an exclusive license to IV Tramadol for the U.S. market from Revogenex Ireland Ltd., or “Revogenex,” pursuant to the Asset Transfer and License Agreement; Fortress subsequently transferred the License Agreement to us. Under the License Agreement, Revogenex was paid a licensing fee of $3.0 million. A $1.0 million milestone payment was due upon NDA submission in December 2019 which was incurred by us. There is also an additional milestone totaling $3.0 million due upon the FDA approval of IV Tramadol. Additional high single-digit to low double-digit royalty payments on net sales of licensed products are due. Royalties will be paid on a product-by-product and country-by-country basis until the expiration in each country of the valid patent claim. In return, Fortress obtained the exclusive worldwide rights to three U.S. patents related to the “Intravenous Administration of Tramadol”: U.S. Patent No. 8,895,622, which issued on November 25, 2014; U.S. Patent No. 9,561,195, which issued on February 7, 2017; and U.S. Patent No. 9,566,253, which issued on February 14, 2017 (all with the exception of Canada, Central America and South America with respect to 50 mg and 100 mg IV Tramadol HCl injections). Additionally, Fortress acquired the rights to an open U.S. Investigational New Drug Application pertaining to IV Tramadol, as well as all supporting documentation and relevant correspondence with the FDA. Further, under the License Agreement, Fortress assumed the rights and obligations of Revogenex under its current manufacturing agreement with Zaklady Farmaceutyczne Polpharma (Polpharma), or the Manufacturing Agreement.
We face potential product liability exposure, and if successful claims are brought against us, we may incur substantial liability for IV Tramadol, BAER-101 (following our acquisition of Baergic Bio) or other product candidates we may license or acquire and may have to limit their commercialization.
The use of IV Tramadol, BAER-101 and any other product candidates we may license or acquire in clinical trials and the sale of any products for which we obtain marketing approval expose us to the risk of product liability claims. For example, we may be sued if any product we develop allegedly causes injury or is found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability or a breach of warranties. Product liability claims might be brought against us by consumers, health care providers or others using, administering or selling our products. If we cannot successfully defend ourselves against these claims, we will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| · | withdrawal of clinical trial participants; |
| · | termination of clinical trial sites or entire trial programs; |
| · | decreased demand for any product candidates or products that we may develop; |
| · | initiation of investigations by regulators; |
| · | impairment of our business reputation; |
| · | costs of related litigation; |
| · | substantial monetary awards to patients or other claimants; |
| · | loss of revenues; |
| · | reduced resources of our management to pursue our business strategy; and |
| · | the inability to commercialize our product candidate or future product candidates. |
We have limited product liability insurance coverage for our clinical trials. However, our insurance coverage may not reimburse us or may not be sufficient to reimburse us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive, and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. When needed, we intend to potentially expand our insurance coverage to include the sale of commercial products if we obtain marketing approval for our product candidate in development, but we may be unable to obtain commercially reasonable product liability insurance for any products approved for marketing. On occasion, large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. A successful product liability claim or series of claims brought against us could cause our stock price to fall and, if judgments exceed our insurance coverage, could decrease our cash and adversely affect our business, financial condition and results of operations.
36
Risks Pertaining to Intellectual Property and Potential Disputes Thereof
If we are unable to obtain and maintain patent protection for our technology and products or if the scope of the patent protection obtained is not sufficiently broad, our competitors could develop and commercialize technology and products similar or identical to ours, and our ability to successfully commercialize our technology and products may be impaired.
Our commercial success will depend in part on obtaining and maintaining patent protection and trade secret protection in the United States with respect to our product candidates and the methods we use to manufacture them, as well as successfully defending these patents and trade secrets against third party challenges. We seek to protect our proprietary position by filing patent applications in the United States and abroad related to our product candidate. We will only be able to protect our technologies from unauthorized use by third parties to the extent that valid and enforceable patents or trade secrets cover them.
The patent prosecution process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. If our licensors or we fail to obtain or maintain patent protection or trade secret protection for IV Tramadol, BAER-101 (following our acquisition of Baergic Bio) or any other product candidate we may license or acquire, third parties could use our proprietary information, which could impair our ability to compete in the market and adversely affect our ability to generate revenues and achieve profitability. Moreover, should we enter into other collaborations we may be required to consult with or cede control to collaborators regarding the prosecution, maintenance and enforcement of our patents. Therefore, these patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. In addition, no consistent policy regarding the breadth of claims allowed in pharmaceutical or biotechnology patents has emerged to date in the United States. The patent situation outside the United States is even more uncertain. The laws of foreign countries may not protect our rights to the same extent as the laws of the United States. For example, European patent law restricts the patentability of methods of treatment of the human body more than United States law does. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after a first filing, or in some cases at all. Therefore, we cannot know with certainty whether we or our licensors were the first to make the inventions claimed in our owned or licensed patents or pending patent applications, or that we were the first to file for patent protection of such inventions. In the event that a third party has also filed a U.S. patent application relating to our product candidates or a similar invention, we may have to participate in interference proceedings declared by the USPTO to determine priority of invention in the United States. The costs of these proceedings could be substantial and it is possible that our efforts would be unsuccessful, resulting in a material adverse effect on our U.S. patent position. As a result, the issuance, scope, validity, enforceability and commercial value of our or any of our licensors’ patent rights are highly uncertain. Our pending and future patent applications may not result in patents being issued which protect our technology or products, in whole or in part, or which effectively prevent others from commercializing competitive technologies and products. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection. For example, the federal courts of the United States have taken an increasingly dim view of the patent eligibility of certain subject matter, such as naturally occurring nucleic acid sequences, amino acid sequences and certain methods of utilizing same, which include their detection in a biological sample and diagnostic conclusions arising from their detection. Such subject matter, which had long been a staple of the biotechnology and biopharmaceutical industry to protect their discoveries, is now considered, with few exceptions, ineligible in the first place for protection under the patent laws of the United States. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in our patents (if any) or in those licensed from third parties.
37
Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and affect the validity, enforceability, scope or defense of our issued patents. On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to United States patent law. These include provisions that affect the way patent applications are prosecuted and may also affect patent litigation. The USPTO recently developed new regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first-to-file provisions, only became effective on March 16, 2013. The Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material, adverse effect on our business and financial condition.
Moreover, we may be subject to a third-party pre-issuance submission of prior art to the USPTO, or become involved in opposition, derivation, reexamination, inter parties review, post-grant review or interference proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, Patent Trial and Appeal Board (“PTAB”) trial, proceeding or litigation could reduce the scope of, render unenforceable, or invalidate, our patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third party patent rights. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates.
Even if our patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our owned or licensed patents by developing similar or alternative technologies or products in a non-infringing manner.
The issuance of a patent does not foreclose challenges to its inventorship, scope, validity or enforceability. Therefore, our owned and licensed patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such product candidates might expire before or shortly after such product candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
The patent rights that we have in-licensed covering the infusion time and pharmacokinetics, or “PK,” profile for IV Tramadol are limited to a specific IV formulation of centrally acting synthetic opioid analgesic, and our market opportunity for this product candidate may be limited by the lack of patent protection for the active ingredient itself and other formulations that may be developed by competitors.
The active ingredients in IV Tramadol have been generic in the United States for a number of years. While we believe that the patent estate covering IV Tramadol (including but not limited to U.S. Patent Nos. 8,895,622; 9,561,195, 9,566,253 9,962,343, 10,406,122, 9,693,949, 9,968,551, 9,980,900, 10,022,321,10,537,521, 10,624,842, 10,751,277, 10,751,278, 10,751,279, 10,646,433, 10,729,644, 10,729,645, and 10,617,635) provides strong protection, our market opportunity would be limited if a generic manufacturer could obtain regulatory approval for another IV formulation of tramadol and commercialize it without infringing our patents.
38
We may become involved in lawsuits to protect or enforce our patents or other intellectual property, which could be expensive, time consuming and unsuccessful.
Competitors may infringe our issued patents or other intellectual property. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time consuming. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their patents. In addition, in a patent infringement proceeding, a court may decide that a patent of ours is invalid or unenforceable, in whole or in part, construe the patent’s claims narrowly or refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of our patents at risk of being invalidated, rendered unenforceable, or interpreted narrowly.
We may become involved in other types of legal proceedings related to our intellectual property that could result in the invalidation or unenforceability of our patents and could be expensive and time consuming, regardless of the outcome.
Any party can challenge the validity of our patents in post-grant proceedings at the PTAB, which include inter partes review and post-grant review proceedings. Although these proceedings are more limited, and therefore are often less expensive, than district court litigation, they can still require substantial resources. If the PTAB finds that our patents are unpatentable, we will be unable to enforce those patents against our competitors. Additionally, our competitors may bring other administrative challenges to our patents before the USPTO, including opposition, derivation, interference, ex parte reexamination, and inter partes reexamination proceedings. These proceedings may prevent our patent applications from issuing, or for patents that are already issued, an unsuccessful outcome will render the patent unenforceable.
If we are sued for infringing intellectual property rights of third parties, it will be costly and time consuming, and an unfavorable outcome in any litigation would harm our business.
Our ability to develop, manufacture, market and potentially sell our product candidates depends upon our ability to avoid infringing the proprietary rights of third parties. Numerous U.S. and foreign patents and pending patent applications, which are owned by third parties, exist in the general fields of pain treatment and central nervous system disorder treatment and cover the use of numerous compounds and formulations in our targeted markets. Because of the uncertainty inherent in any patent or other litigation involving proprietary rights, we and our licensors may not be successful in defending intellectual property claims by third parties, which could have a material adverse effect on our results of operations. Regardless of the outcome of any litigation, defending the litigation may be expensive, time-consuming and distracting to management. In addition, because patent applications can take many years to issue, there may be currently pending applications, unknown to us, which may later result in issued patents that our product candidates may infringe. There could also be existing patents of which we are not aware that one of our product candidates may inadvertently infringe.
There is a substantial amount of litigation involving patent and other intellectual property rights in the biotechnology and biopharmaceutical industries generally. If a third party claims that we infringe on their patents or misappropriated their technology, we could face a number of issues, including:
| · | infringement and other intellectual property claims which, with or without merit, can be expensive and time consuming to litigate and can divert management’s attention from our core business; |
| · | substantial damages for past infringement which we may have to pay if a court decides that our product infringes on a competitor’s patent; |
| · | a court prohibiting us from selling or licensing our product unless the patent holder licenses the patent to us, which it would not be required to do; |
| · | if a license is available from a patent holder, we may have to pay substantial royalties or grant cross licenses to our patents; and |
| · | redesigning our processes so they do not infringe, which may not be possible or could require substantial funds and time. |
39
We may need to license certain intellectual property from third parties, and such licenses may not be available or may not be available on commercially reasonable terms.
A third party may hold intellectual property, including patent rights that are important or necessary to the development and potential commercialization of our product. It may be necessary for us to use the patented or proprietary technology of third parties to potentially commercialize our product, in which case we would be required to obtain a license from these third parties on commercially reasonable terms, or our business could be harmed, possibly materially.
If we fail to comply with our obligations in our intellectual property licenses and funding arrangements with third parties, we could lose rights that are important to our business.
We are currently party to the License Agreement for IV Tramadol. (Please see the section titled “- If we breach the agreement under which we license rights to IV Tramadol, we could lose the ability to continue to develop and potentially commercialize this product candidate.”) The License Agreement will terminate on a product-by-product and country-by-country basis upon the expiration of the last licensed patent right, unless the agreement is earlier terminated. In addition to standard early termination provisions, the License Agreement included provisions allowing early termination by: (i) Revogenex if the FDA did not issue an approval or otherwise issues a “not approvable” notice for the NDA within 15 months after the NDA was filed with the FDA, although this termination right will be tolled if we are using commercially reasonable efforts in our negotiations with the FDA for approval and if we receive a “not approvable” notice, we will have a 15 month period to correct any issues and re-submit the NDA for approval, (ii) us if we reasonably determine prior to NDA approval that the development of IV Tramadol is not economically viable, or (iii) either Revogenex or us (provided we are using or have used commercially reasonable efforts to commercialize IV Tramadol) if, after the third anniversary date of the commercial launch, we fail to achieve annual net sales with respect to IV Tramadol of at least $20 million in any given calendar year, with certain exceptions.
Baergic Bio is similarly party to two license agreements related to BAER-101, one with AstraZeneca AB and another with Cincinnati Children’s Hospital Medical Center. Both license agreements were entered into in December 2019. Baergic Bio acquired an exclusive license from AstraZeneca AB to patent and related intellectual property rights pertaining to its proprietary GABA-A 2,3 positive allosteric modulator, and also acquired from Cincinnati Children’s Hospital Medical Center patent and related intellectual property rights pertaining to GABA inhibition for neurological disorders. Baergic Bio is obligated to use commercially reasonable efforts to develop and commercialize the licensed products in the U.S. and European Union.
In the future, we may become party to licenses that are important for product development and potential commercialization. If we fail to comply with our obligations under current or future license and funding agreements, our counterparties may have the right to terminate these agreements, in which event we might not be able to develop, manufacture or market any product or utilize any technology that is covered by these agreements or may face other penalties under the agreements. Such an occurrence could materially and adversely affect the value of a product candidate being developed under any such agreement or could restrict our drug discovery activities. Termination of these agreements or reduction or elimination of our rights under these agreements may result in our having to negotiate new or reinstated agreements with less favorable terms, or cause us to lose our rights under these agreements, including our rights to important intellectual property or technology.
To the extent we operate in foreign jurisdictions, we may be exposed to increased risk associated with the potential theft of technology and intellectual property.
Our U.S. patents can be enforced against those who make, use, offer to sell, or sell our licensed patented inventions within the U.S., or against those who import our licensed patented inventions within the U.S. We may depend on foreign intellectual property rights to prevent competitors from manufacturing and selling our products outside of the U.S. without our authorization. Foreign laws and regulations may not protect our patent rights and trade secret rights to the same extent as U.S. law. It is also possible that we may be required to compromise protections or waive rights in order to conduct business in a foreign jurisdiction. Such restrictions may limit our ability to profitably compete in those markets.
40
We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is common in the biotechnology and pharmaceutical industry, we employ individuals who were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patent protection for our product candidates or future product candidates, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position, particularly where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. We limit disclosure of such trade secrets where possible but we also seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who do have access to them, such as our employees, our licensors, corporate collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants. Despite these efforts, any of these parties may breach the agreements and may unintentionally or willfully disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. Moreover, if any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
Risks Related to Our Proposed Acquisition of Baergic Bio
Our ability to complete the acquisition of Baergic Bio is subject to closing conditions, including the successful closing of this offering and the receipt of consents and approvals from third parties, which may impose conditions that could adversely affect us or cause the acquisition not to be completed.
Our acquisition of Baergic Bio is subject to a number of closing conditions as specified in the Contribution Agreement entered into with Fortress. These include, among others, (i) the closing of an equity financing by the Company resulting in gross proceeds of no less than $7.5 million, (ii) the agreement by InvaGen to (A) have 100% of its shares in the Company repurchased by the Company and (B) terminate certain of the agreements into which it entered with the Company and/or Fortress in connection with InvaGen’s 2019 equity investment in the Company, which will eliminate certain negative consent rights of InvaGen over the Company and restore certain rights and privileges of Fortress in the Company (all upon terms to be agreed upon with InvaGen), and (iii) the sustained listing of our Common Stock on Nasdaq. Although we have reached an agreement with InvaGen regarding the repurchase of the shares of our Common Stock it holds and the termination of the agreements it entered into with us in 2019, no assurance can be given that all of the required consents and approvals will be obtained or that the closing conditions will be satisfied in a timely manner or at all. Any delay in completing the acquisition could cause the combined company not to realize, or to be delayed in realizing, some or all of the benefits that we expect to achieve. In addition, we can provide no assurance that these conditions will not result in the abandonment or delay of the acquisition. The occurrence of any of these events could have a material adverse effect on our results of operations, cash flows, financial condition and/or the trading price of our Common Stock.
41
We may not achieve the intended benefits of our acquisition of Baergic Bio, and the acquisition may disrupt our current plans or operations.
We may not be able to successfully integrate Baergic Bio’s business and assets or otherwise realize the expected benefits of the transaction, including having a new drug candidate with prospects for FDA approval and commercialization. To realize these anticipated benefits, our business and Baergic Bio’s business must be successfully combined, which is subject to our ability to consolidate operations, corporate cultures and systems and our ability to eliminate redundancies and costs. Difficulties in integrating Baergic Bio into our operations may result in the combined company performing differently than expected, in operational challenges or in the failure to realize anticipated synergies and efficiencies in the expected time frame or at all. The integration of the two companies may result in material challenges, including the diversion of management’s attention from ongoing business concerns; retaining key management and other employees; retaining existing business and operational relationships, including vendors, service providers and other counterparties, and attracting new business and operational relationships; the possibility of faulty assumptions underlying expectations regarding the integration process and associated expenses; consolidating corporate and administrative infrastructures and eliminating duplicative operations; difficulties in the assimilation of employees and corporate cultures; unanticipated issues in integrating information technology, communications and other systems; as well as unforeseen expenses or delays associated with the acquisition. If we are not successful in integrating Baergic Bio’s business and assets or otherwise fail to realize the expected enhanced drug product commercialization opportunities, operating efficiencies, cost savings and other benefits currently anticipated from the Baergic Bio acquisition, our results of operations, cash flows and financial condition may be materially adversely affected.
Whether or not it is completed, the announcement and pendency of the acquisition of Baergic Bio could cause disruptions in our business, which could have an adverse effect on our business and financial results.
Whether or not it is completed, the announcement and pendency of our acquisition of Baergic Bio could cause disruptions in our business and our current and prospective employees may experience uncertainty about their future roles with the combined company, which might adversely affect the ability to retain key employees; uncertainty regarding the completion of the acquisition may cause customers, suppliers, distributors, vendors, strategic partners or others to delay or defer entering into contracts, make other decisions or seek to change or cancel existing business relationships; and the attention of management may be directed toward the completion of the acquisition. If the acquisition is not completed, we will have incurred significant costs and diverted management resources, for which we will have received little or no benefit.
Failure to complete the acquisition of Baergic Bio could negatively impact our stock price and the future business and financial results.
If the acquisition of Baergic Bio is not completed for any reason, our ongoing business may be adversely affected, and without realizing any benefits of having completed the acquisition, we would be subject to a number of risks, including the following:
| · | we may experience negative reactions from the financial markets, including negative impacts on our stock price; |
| · | we may experience negative reactions from our employees; |
| · | we may experience adverse impacts on our relationships with vendors and industry contracts which could adversely affect our respective results of operations and financial condition; |
| · | we will be required to pay certain costs relating to the acquisition, whether or not the acquisition is completed; and |
42
| · | we may have expended substantial commitments of time and resources on matters relating to the acquisition (including integration planning), which would otherwise have been devoted to day-to-day operations and other opportunities that may have been beneficial to us as a standalone company. |
In addition to the above risks, if the Contribution Agreement is terminated and our board of directors instead seeks an alternative transaction, our stockholders cannot be certain that we will be able to find another party willing to engage in a transaction on more attractive terms than those contemplated by the Contribution Agreement. Accordingly, if our acquisition of Baergic Bio is not completed, these risks may materialize and may adversely affect our business, financial condition, results of operations and stock price.
We are expected to incur substantial expenses related to the acquisition of Baergic Bio and its affiliates and the integration of its business with ours.
We expect to incur substantial expenses in connection with the integration of our business with Baergic Bio. There are a number of processes, policies, procedures, operations, technologies and systems that must be integrated, including purchasing, accounting and finance, sales, payroll, pricing, revenue management, marketing and benefits. Some of these costs will be non-recurring expenses related to the acquisition itself, including legal and accounting costs and systems consolidation costs. We may also incur additional costs to attract, motivate or retain management personnel and other key employees. We have incurred and will continue to incur acquisition fees and costs related to formulating integration plans for the combined business, and the execution of these plans may lead to additional unanticipated costs.
The unaudited pro forma combined financial statements included in this prospectus are based on a number of preliminary estimates and assumptions and the actual results of operations and financial position of the combined company after the acquisition may differ materially.
The unaudited pro forma combined financial statements in this prospectus are based on the historical financial statements of the Company and Baergic Bio after giving effect to the acquisition and the assumptions and adjustments as discussed in the section titled “Unaudited Pro Forma Financial Statements” of this prospectus.
Such pro forma condensed financial statements are subject to numerous risks and uncertainties, rely on a number of assumptions and are not a guarantee of future performance. The assumptions used in preparing the pro forma combined financial statements may not prove to be accurate, and other factors may affect the combined company’s financial condition or results of operations following the proposed transactions contemplated by the Contribution Agreement. The results indicated in the unaudited pro forma combined financial information may not be realized and future financial results may materially vary from the unaudited pro forma combined financial statements. See the section titled “Unaudited Pro Forma Combined Financial Statements” beginning on page 51 of this prospectus.
The market price of our Common Stock following the acquisition of Baergic Bio may decline as a result of the transaction.
The market price of our Common Stock may decline as a result of our acquisition of Baergic Bio and its affiliates for a number of reasons, including if:
| · | investors react negatively to the prospects of the combined company’s business and financial condition following the acquisition; |
| · | the effect of the acquisition on the combined company’s business and prospects is not consistent with the expectations of financial or industry analysts; or |
| · | the combined company does not achieve the perceived benefits of the acquisition as rapidly or to the extent anticipated by management and the Company’s investors, or at all. |
Risks Related to this Offering
If the price of our Common Stock fluctuates significantly, your investment could lose value.
43
Although our Common Stock is listed on Nasdaq, we cannot assure you that an active public market will continue for our Common Stock. If an active public market for our Common Stock does not continue, the trading price and liquidity of our Common Stock will be materially and adversely affected. If there is a thin trading market or “float” for our stock, the market price for our Common Stock may fluctuate significantly more than the stock market as a whole. Without a large float, our Common Stock would be less liquid than the stock of companies with broader public ownership and, as a result, the trading prices of our Common Stock may be more volatile. In addition, in the absence of an active public trading market, investors may be unable to liquidate their investment in us. Furthermore, the stock market is subject to significant price and volume fluctuations, and the price of our Common Stock could fluctuate widely in response to several factors, including:
| · | our quarterly or annual operating results; |
| · | changes in our earnings estimates; |
| · | investment recommendations by securities analysts following our business or our industry; |
| · | additions or departures of key personnel; |
| · | our failure to achieve operating results consistent with securities analysts’ projections; |
| · | changes in industry, general market or economic conditions; and |
| · | our failure to complete the acquisition of Baergic Bio. |
The stock market has experienced extreme price and volume fluctuations in recent years that have significantly affected the quoted prices of the securities of many companies, including companies in our industry. The changes often appear to occur without regard to specific operating performance. The price of our Common Stock could fluctuate based upon factors that have little or nothing to do with our company and these fluctuations could materially reduce our stock price.
We will have broad discretion in the use of proceeds of this offering designated for working capital and general corporate purposes.
We intend to use the net proceeds from this offering to repurchase all of the shares of our Common Stock held by InvaGen for a purchase price of $3 million under the terms of the Share Repurchase Agreement we entered into with InvaGen in July 2022, with any funds remaining thereafter for general corporate purposes and working capital requirements, which may include, among other things, the advancement of BAER-101 (following the closing of our acquisition of Baergic Bio) and IV Tramadol to obtain regulatory approval from the FDA. Additionally, our management will have broad discretion over the use and investment of the net proceeds of this offering. Accordingly, investors in this offering have only limited information concerning our management’s specific intentions and will need to rely upon the judgment of our management with respect to the use of proceeds.
We do not intend to pay dividends on our Common Stock, so any returns will be limited to increases, if any, in our stock’s value. Your ability to achieve a return on your investment will depend on appreciation, if any, in the price of our Common Stock.
We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Any future determination to declare dividends will be made at the discretion of our board of directors and will depend on, among other factors, our financial condition, operating results, capital requirements, general business conditions and other factors that our board of directors may deem relevant. Any return to stockholders will therefore be limited to the appreciation in the value of their stock, if any.
The warrants are speculative in nature.
The warrants included in the units and pre-funded units offered hereby do not confer any rights of Common Stock ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of our Common Stock at a fixed price. Specifically, commencing on the date of issuance, holders of the warrants may exercise their right to acquire the shares of our Common Stock and pay an exercise price of $ , equal to the public offering price per unit or pre-funded warrant. Moreover, following this offering, the market value of the warrants is uncertain and there can be no assurance that the market value of the warrants will equal or exceed their exercise price. Furthermore, each warrant will expire five years from the original issuance date. In the event the price of our Common Stock does not exceed the exercise price of the warrants during the period when the warrants are exercisable, the warrants may not have any value. There is no established public trading market for warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend to apply to list the warrants on any securities exchange or nationally recognized trading system, including Nasdaq. Without an active market, the liquidity of the warrants will be limited.
44
Holders of the warrants or pre-funded warrants will have no rights as a common stockholder until they acquire shares of our Common Stock.
Until you acquire shares of our Common Stock upon exercise of your warrants or pre-funded warrants, you will have no rights with respect to shares of Common Stock issuable upon exercise of such warrants. Upon exercise of your warrants or pre-funded warrants, you will be entitled to exercise the rights of a holder of our Common Stock as to the security exercised only as to matters for which the record date occurs after the exercise.
Provisions of the warrants and pre-funded warrants offered by this prospectus could discourage an acquisition of us by a third party.
In addition to the provisions of our amended and restated certificate of incorporation and bylaws discussed elsewhere in this prospectus, certain provisions of the warrants and pre-funded warrants offered by this prospectus could make it more difficult or expensive for a third party to acquire us. The warrants and pre-funded warrants prohibit us from engaging in certain transactions constituting “fundamental transactions” unless, among other things, the surviving entity assumes our obligations under the warrants. These and other provisions of the warrants and pre-funded warrants offered by this prospectus could prevent or deter a third party from acquiring us even where the acquisition could be beneficial to you.
If you purchase shares of our Common Stock included as part of the units in this offering, you will incur immediate and substantial dilution in the book value of your shares.
Investors purchasing shares of our Common Stock included as part of the units in this offering will pay a price per unit that substantially exceeds the pro forma as adjusted net tangible book value per share. As a result, investors purchasing shares of our Common Stock included as part of the units in this offering will incur immediate dilution of $1.15 per share, representing the difference between the public offering price of $3.30 per unit and our pro forma as adjusted net tangible book value per share as of June 30, 2022. To the extent outstanding options or warrants to purchase our Common Stock are exercised, new investors may incur further dilution. For more information on the dilution you may experience as a result of investing in this offering, see the section of this prospectus entitled “Dilution.”
If we sell Common Stock or preferred stock in future financings, stockholders may experience immediate dilution and, as a result, our stock price may decline.
We may from time to time issue additional shares of Common Stock or preferred stock at a discount from the current trading price of our Common Stock. As a result, our stockholders would experience immediate dilution upon the purchase of any shares sold at such discount. In addition, as opportunities present themselves, we may enter into financing or similar arrangements in the future, including the issuance of debt securities, Common Stock or preferred stock. If we issue Common Stock or securities convertible into Common Stock, the holders of our Common Stock would experience additional dilution and, as a result, our stock price may decline.
General Risk Factors
Our business and operations could be adversely affected by the effects of health epidemics, including the ongoing COVID-19 pandemic.
45
Any potential future clinical trials may experience delays in patient enrollment, potentially due to prioritization of hospital resources toward the COVID-19 pandemic, or concerns among patients about participating in clinical trials during a public health emergency. The COVID-19 pandemic is affecting the operations of government entities, such as the FDA, as well as contract research organizations, third-party manufacturers, and other third-parties upon whom we rely. As a result of “shelter-in-place” orders, quarantines or similar orders or restrictions to control the spread of COVID-19, many companies, including our own, implemented work-from-home policies for their employees during 2020, 2021 and into 2022. The effects of these stay-at-home orders and work-from-home policies may be negatively impacting productivity, resulting in delays in our timelines. The extent of the impact on our operations depends in part on whether governments and businesses reinstate these restrictions as a result of a rising surge in COVID-19 cases or a new variant of the virus. These and similar disruptions in our operations could negatively impact our business, operating results and financial condition, however, as of the date of this prospectus, we have not experienced a significant impact on our business resulting from government restrictions on the movement of people, goods, and services.
The global pandemic of COVID-19 continues to evolve rapidly, and the ultimate impact of the COVID-19 pandemic or a similar health epidemic is highly uncertain and subject to change. We do not yet know the full impact of potential delays or effects on our business, our ability to access the capital markets, or supply chains or on the global economy as a whole. However, these effects could have a material impact on our operations, and we will continue to monitor the COVID-19 situation closely.
Our results of operations and liquidity needs could be materially negatively affected by market fluctuations and economic downturn.
Our results of operations could be materially negatively affected by economic conditions generally, both in the United States and elsewhere around the world. Continuing concerns over inflation, energy costs, geopolitical issues, including the invasion of Ukraine by military forces of the Russian Federation, the availability and cost of credit, the U.S. mortgage market and residential real estate market in the United States have contributed to increased volatility and diminished expectations for the economy and the markets going forward. These factors, combined with volatile oil prices, declining business and consumer confidence and increased interest rate, have precipitated an economic recession and fears of a possible depression. Domestic and international equity markets continue to experience heightened volatility and turmoil. These events and the continuing market upheavals may have an adverse effect on us. In the event of a continuing market downturn, our results of operations could be adversely affected by those factors in many ways, including making it more difficult for us to raise funds if necessary, and our stock price may further decline.
We will continue to incur significant increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives.
We are a listed and traded public company. As a public company, we incur significant legal, accounting and other expenses under the Sarbanes-Oxley Act of 2002, as well as rules subsequently implemented by the SEC and the rules of the Nasdaq Stock Market, on which our Common Stock is listed. These rules impose various requirements on public companies, including requiring establishment and maintenance of effective disclosure and financial controls and appropriate corporate governance practices. Our management and other personnel have devoted and will continue to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations increase our legal and financial compliance costs and make some activities more time-consuming and costly. For example, these rules and regulations make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as executive officers.
46
The Sarbanes-Oxley Act of 2002 requires, among other things, that we maintain effective internal controls for financial reporting and disclosure controls and procedures. As a result, we are required to periodically perform an evaluation of our internal controls over financial reporting to allow management to report on the effectiveness of those controls, as required by Section 404 of the Sarbanes-Oxley Act. However, while we remain either a non-accelerated filer and/or an emerging growth company, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To achieve compliance with Section 404 within the prescribed period, we have engaged in a process to document and evaluate our internal control over financial reporting. These efforts to comply with Section 404 and related regulations have required, and continue to require, the commitment of significant financial and managerial resources. While we anticipate maintaining the integrity of our internal controls over financial reporting and all other aspects of Section 404, we cannot be certain that a material weakness will not be identified when we test the effectiveness of our control systems in the future. If a material weakness is identified, we could be subject to sanctions or investigations by the SEC or other regulatory authorities, which would require additional financial and management resources, costly litigation or a loss of public confidence in our internal controls, which could have an adverse effect on the market price of our stock.
Our business and operations would suffer in the event of system failures.
Despite the implementation of security measures, our internal computer systems are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Any system failure, accident or security breach that causes interruptions in our operations could result in a material disruption of our drug development programs. For example, the loss of clinical trial data from completed clinical trials for IV Tramadol could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach results in a loss or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we may incur liability and the further development of our product candidate may be delayed.
The occurrence of a catastrophic disaster could damage our facilities beyond insurance limits or we could lose key data which could cause us to curtail or cease operations.
We are vulnerable to damage and/or loss of vital data from natural disasters, such as earthquakes, tornadoes, power loss, fire, health epidemics and pandemics, floods and similar events, as well as from accidental loss or destruction. If any disaster were to occur, our ability to operate our businesses could be seriously impaired. We have property, liability and business interruption insurance that may not be adequate to cover losses resulting from disasters or other similar significant business interruptions, and we do not plan to purchase additional insurance to cover such losses due to the cost of obtaining such coverage. Any significant losses that are not recoverable under our insurance policies could seriously impair our business, financial condition and prospects. Any of the aforementioned circumstances, including without limitation the emerging COVID-19 virus, may also impede our employees’ and consultants’ abilities to provide services in-person and/or in a timely manner; hinder our ability to raise funds to finance our operations on favorable terms or at all; and trigger effectiveness of “force majeure” clauses under agreements with respect to which we receive goods and services, or under which we are obligated to achieve developmental milestones on certain timeframes. Disputes with third parties over the applicability of such “force majeure” clauses, or the enforceability of developmental milestones and related extension mechanisms in light of such business interruptions, may arise and may become expensive and time-consuming.
We may become involved in securities class action litigation that could divert management’s attention and harm our business.
The stock markets have from time to time experienced significant price and volume fluctuations that have affected the market prices for the Common Stock of biotechnology and pharmaceutical companies. These broad market fluctuations may cause the market price of our stock to decline. In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biotechnology and biopharmaceutical companies have experienced significant stock price volatility in recent years and due to the significant stock price decline we experienced following the announcement of the First CRL. We may become involved in this type of litigation in the future. Litigation often is expensive and diverts management’s attention and resources, which could adversely affect our business.
47
The following table sets forth our cash and capitalization as of June 30, 2022, as follows:
| · | on an actual basis; |
| · | on an as adjusted basis to reflect the issuance and sale by us of 3,636,365 units in this offering at a public offering price of $3.30 per unit and as adjusted to account for our one-for-fifteen reverse stock split that was effected on September 22, 2022), after deducting the estimated offering expenses payable by us. |
The as adjusted information below is illustrative only, and our capitalization following the completion of this offering will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. You should read this information in conjunction with our financial statements and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section in our Form 10-K, which is incorporated by reference in this prospectus.
| June 30, 2022 | ||||||||
| (unaudited) | ||||||||
| ($ in thousands) | Actual | As Adjusted | ||||||
| Cash and cash equivalents | $ | 890 | $ | 8,303 | ||||
| Stockholders’ Equity (Deficit) | ||||||||
| Preferred Stock ($0.0001 par value), 2,000,000 shares authorized | ||||||||
| Class A Preferred Stock – 250,000 shares issued and outstanding | — | — | ||||||
| Common Stock ($0.0001 par value), 20,000,000 shares authorized | ||||||||
| Common stock – 1,475,652 issued and outstanding actual; 5,112,017 as adjusted | 2 | 2 | ||||||
| Additional paid-in capital | 81,060 | 91,840 | ||||||
| Accumulated deficit | (80,464 | ) | (80,464 | ) | ||||
| Total Stockholders’ Equity (Deficit) | 598 | 11,011 | ||||||
| Total Capitalization | $ | 598 | $ | 11,011 | ||||
The number of shares of Common Stock to be outstanding after this offering is based on 1,475,652 shares of our Common Stock outstanding as of June 30, 2022, and:
| · | excludes 996 shares of Common Stock issuable upon exercise of outstanding warrants having a weighted-average exercise price of $10.05 per share; |
48
| · | excludes 21,415 shares of Common Stock issuable upon the vesting and settlement of outstanding restricted stock award/units; |
| · | excludes 122,489 shares of Common Stock reserved for issuance and available for future grant under our 2015 Incentive Plan; and |
| · | excludes 3,636,365 shares of Common Stock issuable upon exercise of the warrants included in the units; and |
| · | assumes no exercise by the underwriter of its over-allotment option to purchase additional securities. |
We currently intend to retain all available funds and any future earnings to fund the growth and development of our business. We have never declared or paid any cash dividends on our capital stock. We do not intend to pay cash dividends on our Common Stock in the foreseeable future. Investors should not purchase our common stock with the expectation of receiving cash dividends.
Any future determination to declare dividends will be made at the discretion of our board of directors and will depend on our financial condition, operating results, capital requirements, general business conditions, and other factors that our board of directors may deem relevant.
We estimate that we will receive net proceeds from this offering of approximately $10.4 million, or approximately $12.1 million, if the underwriter exercises its over-allotment option in full, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us and assuming no exercise of the warrants included in the units or pre-funded units. We will only receive additional proceeds from the exercise of the warrants included in the units and pre-funded units we are selling in this offering if the warrants are exercised for cash.
We currently estimate that we will use $3 million of the net proceeds from this offering to repurchase all of the shares of our Common Stock held by InvaGen under the terms of the Share Repurchase Agreement we entered into with InvaGen in July 2022. We intend to use the remainder of the net proceeds for general corporate purposes and working capital requirements, which may include, among other things, the advancement of BAER-101 (following the closing of our acquisition of Baergic Bio) and IV Tramadol to obtain regulatory approval from the FDA.
Our expected use of proceeds from this offering represents our current intentions based on our recent plans and business condition. As of the date of this prospectus, we cannot predict with certainty all of the particular uses for the proceeds to be received upon the completion of this offering. We may use a portion of the proceeds to pursue selective strategic investment and acquisition opportunities to expand and support our business growth. Although we have no specific agreements, commitments, or understandings with respect to any such activity or acquisition, we evaluate these opportunities and engage in related discussions with other companies or their shareholders from time to time. The amounts and timing of our actual expenditures will depend on numerous factors, such as the timing and success of any future clinical trials and preclinical studies, as well as subsequent regulatory submissions for our licensed products, the feasibility of any acquisitions or other investments, the amounts of proceeds actually raised in this offering and the amount of cash generated by our operations. Because we operate in a very dynamic and highly competitive industry, the actual use of proceeds may differ substantially from the ranges indicated above. Our management will have broad discretion to allocate the net proceeds from this offering.
Pending the use of the net proceeds from this offering, we may invest them in short-term and medium-term interest-bearing instruments.
49
Purchasers of the securities offered by this prospectus will suffer immediate and substantial dilution in the net tangible book value per share of the Common Stock included in the units they purchase. Net tangible book value per share represents the amount of total tangible assets less total liabilities, divided by the number of shares of our Common Stock outstanding as of June 30, 2022, effected for the one-for-fifteen reverse stock split. Our net tangible book value as of June 30, 2022 was approximately $0.6 million, or $0.41 per share of our Common Stock.
Dilution in net tangible book value per share represents the difference between the amount per share paid by purchasers in this offering and the net tangible book value per share of our Common Stock immediately after this offering. After giving effect to the sale of units in this offering at a public offering price of $3.30 per unit, and after deducting the underwriting discount and the estimated expenses payable by us, our net tangible book value as of June 30, 2022 would have been approximately $11.0 million, or $2.15 per share of Common Stock. This represents an immediate increase in net book value of $1.74 per share to our existing stockholders and an immediate dilution in net tangible book value of $1.15 per share to new investors participating in this offering.
The following table illustrates this calculation on a per share basis:
| Offering price per share | $ | 3.30 | ||||||
| Net tangible book value per share as of June 30, 2022 | $ | 0.41 | ||||||
| Increase per share attributable to the offering | $ | 1.74 | ||||||
| As-adjusted net tangible book value per share after giving effect to the offering | $ | 2.15 | ||||||
| Dilution in net tangible book value per share to new investors | $ | 1.15 |
The number of shares of Common Stock to be outstanding after this offering is based on 1,475,652 shares of our Common Stock outstanding as of June 30, 2022, and:
| · | excludes 996 shares of Common Stock issuable upon exercise of outstanding warrants having a weighted-average exercise price of $10.05 per share; |
| · | excludes 21,415 shares of Common Stock issuable upon the vesting and settlement of outstanding restricted stock award/units; |
| · | excludes 122,489 shares of Common Stock reserved for issuance and available for future grant under our 2015 Incentive Plan; and |
| · | excludes 3,636,365 shares of Common Stock issuable upon exercise of the warrants included in the units; and |
| · | assumes no exercise by the underwriter of its over-allotment option to purchase additional securities. |
If the underwriter exercises in full its option to purchase 545,454 additional shares of Common Stock (and no purchase of pre-funded warrants), then the as-adjusted net tangible book value after this offering would be $2.24 per share, representing an increase in net tangible book value of $1.83 per share to existing stockholders and immediate dilution in net tangible book value of $1.06 per share to purchasers in this offering.
50
The selected financial data is presented to provide the effects of the one-for-fifteen reverse stock split on the historical financial position and results of Avenue. Our historical consolidated financial information has been derived from the consolidated audited and unaudited financial statements of the Company and accompanying notes to the financial statements incorporated by reference into this prospectus. Our historical results are not necessarily indicative of results that should be expected in any future period, and our results for any interim period are not necessarily indicative of results that should be expected for any full year.
As of June 30, 2022 | As of March 31, 2022 | As of December 31, 2021 | As of December 31, 2020 | |||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||
| Balance Sheet Data: | ||||||||||||||||
| ASSETS | ||||||||||||||||
| Current Assets: | ||||||||||||||||
| Cash and cash equivalents | $ | 890 | $ | 1,833 | $ | 3,763 | $ | 3,132 | ||||||||
| Other receivables – related party | - | - | 90 | - | ||||||||||||
| Prepaid expenses and other current assets | 115 | 123 | 107 | 113 | ||||||||||||
| Total current assets | 1,005 | 1,956 | 3,960 | 3,245 | ||||||||||||
| Total Assets | $ | 1,005 | $ | 1,956 | $ | 3,960 | $ | 3,245 | ||||||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||||||||||
| Current Liabilities: | ||||||||||||||||
| Accounts payable and accrued expenses | 397 | 746 | 451 | 857 | ||||||||||||
| Accounts payable and accrued expenses - related party | 10 | 51 | 58 | 29 | ||||||||||||
| Total current liabilities | 407 | 797 | 509 | 886 | ||||||||||||
| Total Liabilities | 407 | 797 | 509 | 886 | ||||||||||||
| Commitments and Contingencies | ||||||||||||||||
| Stockholders’ Deficit | ||||||||||||||||
| Preferred Stock ($0.0001 par value), 2,000,000 shares authorized and 250,000 shares outstanding as of June 30, 2022, March 31, 2002, and December 31, 2021 and 2020 | – | – | – | – | ||||||||||||
| Common Stock ($0.0001 par value), 20,000,000 shares authorized and 1,475,652, 1,745,652, 1,405,977, 1,116,520 shares issued and outstanding as of June 30, 2022, March 31, 2022 and December 31, 2021 and 2020, respectively | 2 | 2 | 2 | 2 | ||||||||||||
| Additional paid-in capital | 81,060 | 81,017 | 80,448 | 75,625 | ||||||||||||
| Accumulated deficit | (80,464 | ) | (79,860 | ) | (76,999 | ) | (73,268 | ) | ||||||||
| Total Stockholders’ Deficit | 598 | 1,159 | 3,451 | 2,359 | ||||||||||||
| Total Liabilities and Stockholders’ Deficit | $ | 1,005 | $ | 1,956 | $ | 3,960 | $ | 3,245 | ||||||||
51
| Three Months Ended | Three Months Ended | Six Months Ended | Six Months Ended | |||||||||||||
| June 30, 2022 | June 30, 2021 | June 30, 2022 | June 30, 2021 | |||||||||||||
| (unaudited) | (unaudited) | (unaudited) | (unaudited) | |||||||||||||
| Statement of Operations Data: | ||||||||||||||||
| Expenses | ||||||||||||||||
| Research and development | $ | 151 | $ | 328 | $ | 1,959 | $ | 586 | ||||||||
| General and administrative | 454 | 623 | 1,509 | 1,366 | ||||||||||||
| Total expenses | 605 | 951 | 3,468 | 1,952 | ||||||||||||
| Operating loss | (605 | ) | (951 | ) | (3,468 | ) | (1,952 | ) | ||||||||
| Other income (expense) | ||||||||||||||||
| Interest income | 1 | 2 | 3 | 5 | ||||||||||||
| Net loss | $ | (604 | ) | $ | (949 | ) | $ | (3,465 | ) | $ | (1,947 | ) | ||||
| Basic and diluted net loss per share | $ | (0.41 | ) | $ | (0.86 | ) | $ | (2.42 | ) | $ | (1.76 | ) | ||||
| Basic and diluted weighted average shares outstanding | 1,461,067 | 1,103,754 | 1,429,282 | 1,103,754 | ||||||||||||
| Three Months Ended | Three Months Ended | Year Ended | Year Ended | |||||||||||||
| March 31, 2022 | March 31, 2021 | December 31, 2021 | December 31, 2020 | |||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||
| Statement of Operations Data: | ||||||||||||||||
| Expenses | ||||||||||||||||
| Research and development | $ | 1,808 | $ | 258 | $ | 1,254 | $ | 2,866 | ||||||||
| General and administrative | 1,055 | 743 | 2,484 | 2,347 | ||||||||||||
| Total expenses | 2,863 | 1,001 | 3,738 | 5,213 | ||||||||||||
| Operating loss | (2,863 | ) | (1,001 | ) | (3,738 | ) | (5,213 | ) | ||||||||
| Other income (expense) | ||||||||||||||||
| Interest income | 2 | 3 | 7 | 62 | ||||||||||||
| Net loss | $ | (2,861 | ) | $ | (998 | ) | $ | (3,731 | ) | $ | (5,151 | ) | ||||
| Basic and diluted net loss per share | $ | (2.05 | ) | $ | (0.90 | ) | $ | (3.29 | ) | $ | (4.68 | ) | ||||
| Basic and diluted weighted average shares outstanding | 1,397,145 | 1,103,754 | 1,113,170 | 1,100,429 | ||||||||||||
52
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL STATEMENTS
The unaudited pro forma condensed combined consolidated financial information is presented to illustrate the estimated effects of the acquisition of Baergic Bio by Avenue based on the historical financial position and results of operations of Avenue and Baergic Bio. It is presented as follows:
| ● | The unaudited pro forma condensed combined consolidated balance sheet as of June 30, 2022 was prepared based on (i) the historical unaudited condensed consolidated balance sheet of Avenue as of June 30, 2022 and (ii) the historical unaudited balance sheet of Baergic Bio as of June 30, 2022. |
| ● | The unaudited pro forma condensed combined consolidated statement of operations for the years ended December 31, 2021, and 2020 were prepared based on (i) the historical audited consolidated statement of operations of Avenue for the years ended December 31, 2021, and 2020 and (ii) the historical audited statement of operations of Baergic Bio for the years ended December 31, 2021, and 2020. | |
| ● | The unaudited pro forma condensed combined consolidated statement of operations for the six months ended June 30, 2022 was prepared based on (i) the historical unaudited condensed consolidated statement of operations of Avenue for the six months ended June 30, 2022 and (ii) the historical unaudited statement of operations of Baergic Bio for the six months ended June 30, 2022. |
Our historical consolidated financial information has been derived from the consolidated audited and unaudited financial statements of the Company and accompanying notes to the financial statements incorporated by reference into this prospectus. The historical consolidated financial information of Baergic Bio have been derived from the consolidated audited and unaudited financial statements of Baergic Bio and accompanying notes to the financial statements included in this prospectus.
The unaudited pro forma condensed combined consolidated financial information was prepared in accordance with Article 11 of SEC Regulation S-X. See the accompanying notes to the Unaudited Pro Forma Consolidated Financial Information for a discussion of assumptions made.
The transaction will be accounted for as a transaction between entities under common control, such that Avenue will recognize the assets and liabilities of Baergic Bio received in the transaction at their historical carrying amounts, as reflected in the historical consolidated financial statements of Baergic Bio. No Goodwill or intangibles will be recognized. As such, Avenue will recognize the contribution on a prospective basis from the transaction closing date. The unaudited pro forma condensed combined financial information set forth below primarily gives effect to the following:
| ● | the contribution by Fortress to Avenue of Baergic Bio and the forgiveness of certain intercompany balances; | |
| ● | the issuance of equity securities; | |
| ● | the payment to InvaGen to repurchase Company shares; | |
| ● | the one-for-fifteen reverse stock split effected by Avenue immediately prior to the closing of this offering, and | |
| ● | transaction costs incurred in connection with the transaction. |
Assumptions underlying the pro forma adjustments are described in the accompanying notes, which should be read in conjunction with the unaudited pro forma condensed combined consolidated financial information. The unaudited pro forma condensed combined consolidated balance sheet data gives effect to the transaction as if it had occurred on June 30, 2022. The unaudited pro forma condensed combined consolidated statements of operations data for the six months ended June 30, 2022 and the years ended December 31, 2021, and 2020 give effect to the transaction as if it had occurred on January 1, 2020.
The unaudited pro forma condensed combined financial information has been presented for informational purposes only and is not necessarily indicative of what the combined company’s financial position or results of operations actually would have been had Avenue and Baergic Bio been a combined company as of the dates indicated. In addition, the unaudited pro forma condensed combined financial information does not purport to project the future financial position or operating results of the combined company. The historical consolidated financial information has been adjusted in the accompanying unaudited pro forma condensed combined consolidated financial information to give effect to unaudited pro forma events.
53
UNAUDITED PRO FORMA CONDENSED COMBINED
CONSOLIDATED BALANCE SHEET
AS OF JUNE 30, 2022
(Amounts in thousands)
| June 30, 2022 | June 30, 2022 | June 30, 2022 | ||||||||||||||||
| Transaction | ||||||||||||||||||
| Avenue | Baergic | Accounting | Pro Forma | |||||||||||||||
| (Historical) | (Historical) | Adjustments | Notes | Combined | ||||||||||||||
| ASSETS | ||||||||||||||||||
| Current assets | ||||||||||||||||||
| Cash and cash equivalents | $ | 890 | $ | 11 | $ | 12,000 | 5(a) | 8,314 | ||||||||||
| (1,020 | ) | 5(b) | ||||||||||||||||
| (3,000 | ) | 5(c) | ||||||||||||||||
| (567 | ) | 5(e) | ||||||||||||||||
| Prepaid expenses and other assets | 115 | - | - | 115 | ||||||||||||||
| Total current assets | 1,005 | 11 | 7,413 | 8,429 | ||||||||||||||
| Total assets | $ | 1,005 | $ | 11 | $ | 7,413 | $ | 8,429 | ||||||||||
| LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIT) | ||||||||||||||||||
| Current liabilities | ||||||||||||||||||
| Accounts payable and accrued expenses | $ | 397 | $ | 19 | $ | - | 416 | |||||||||||
| Accounts payable and accrued expenses - related party | 10 | 1,270 | (1,270 | ) | 5(d) | 10 | ||||||||||||
| Accrued interest – related party | - | 722 | (722 | ) | - | |||||||||||||
| Notes payable | - | 4,074 | (4,074 | ) | - | |||||||||||||
| Total current liabilities | 407 | 6,085 | (6,066 | ) | 426 | |||||||||||||
| Total liabilities | 407 | 6,085 | (6,066 | ) | 426 | |||||||||||||
| Commitments and Contingencies | ||||||||||||||||||
| Stockholders' equity (deficit) | ||||||||||||||||||
| Preferred stock Avenue Preferred Stock ($0.0001 par value), 2,000,000 shares authorized and 250,000 shares outstanding as of June 30, 2022) Baergic Preferred Stock ($0.0001 par value), 2,000,000 shares authorized and 250,000 shares outstanding as of June 30, 2022) | - | - | ||||||||||||||||
| Common stock Avenue Common Stock ($0.0001 par value), 20,000,000 shares authorized and 1,475,652 shares outstanding as of June 30, 2022) | 2 | 1 | - | 5(a) | - | |||||||||||||
| Baergic Common Stock ($0.0001 par value), 50,000,000 shares authorized and 14,297,173 shares outstanding as of June 30, 2022) | - | - | (3 | ) | 5(f) | |||||||||||||
| Additional paid-in capital | 81,060 | 141 | 12,000 | 5(a) | 97,052 | |||||||||||||
| (1,020 | ) | 5(b) | ||||||||||||||||
| (3,000 | ) | 5(c) | ||||||||||||||||
| 1,270 | 5(d) | |||||||||||||||||
| (567 | ) | 5(e) | ||||||||||||||||
| 2,369 | 5(f) | |||||||||||||||||
| 3 | 5(g) | |||||||||||||||||
| 4,796 | 5(h) | |||||||||||||||||
| Accumulated deficit | (80,464 | ) | (6,216 | ) | - | (86,680 | ) | |||||||||||
| Total stockholders' equity attributed to Company | 598 | (6,074 | ) | 15,848 | 10,372 | |||||||||||||
| Non-controlling interest | - | - | (2,369 | ) | 5(f) | (2,369 | ) | |||||||||||
| Total stockholders' equity (deficit) | 598 | (6,074 | ) | 13,479 | 8,003 | |||||||||||||
| Total liabilities and stockholders' equity (deficit) | $ | 1,005 | $ | 11 | $ | 7,413 | $ | 8,429 | ||||||||||
See accompanying notes to the unaudited pro forma condensed combined consolidated financial information.
Avenue Common Stock shares authorized and outstanding are shown with the effect of the reduction in authorized shares and one-for-fifteen reverse-split that took effect as of September 22, 2022.
54
UNAUDITED PRO FORMA CONDENSED COMBINED
CONSOLIDATED STATEMENT OF OPERATIONS
FOR THE SIX MONTHS ENDED JUNE 30, 2022
(Amounts in thousands, except share and per share amounts)
| Six Months Ended | Six Months Ended | Six Months Ended | ||||||||||||||||
| June 30, 2022 | June 30, 2022 | June 30, 2022 | ||||||||||||||||
| Transaction | ||||||||||||||||||
| Avenue | Baergic | Accounting | Pro Forma | |||||||||||||||
| (Historical) | (Historical) | Adjustments | Notes | Combined | ||||||||||||||
| Expenses | ||||||||||||||||||
| Research and development | $ | 1,959 | $ | 166 | $ | - | $ | 2,125 | ||||||||||
| General and administrative | 1,509 | 206 | 567 | 5(e) | 2,282 | |||||||||||||
| Total expenses | 3,468 | 372 | 567 | 4,407 | ||||||||||||||
| Operating loss | (3,468 | ) | (372 | ) | (567 | ) | (4,407 | ) | ||||||||||
| Other income (expense) | ||||||||||||||||||
| Interest income | 3 | - | - | 3 | ||||||||||||||
| Interest expense – related party | - | (165 | ) | - | (165 | ) | ||||||||||||
| Total other income (expense) | 3 | (165 | ) | - | (162 | ) | ||||||||||||
| Net loss | $ | (3,465 | ) | $ | (537 | ) | $ | (567 | ) | $ | (4,569 | ) | ||||||
| Basic and diluted net loss per share | $ | (2.42 | ) | $ | (0.98 | ) | ||||||||||||
| Basic and diluted weighted average shares outstanding | 1,429,282 | 4,676,759 | ||||||||||||||||
See accompanying notes to the unaudited pro forma condensed combined consolidated financial information.
Avenue basic and diluted weighted average shares outstanding and basic and diluted net loss per share are shown with the effect of one-for-fifteen reverse-split that took effect as of September 22, 2022.
55
UNAUDITED PRO FORMA CONDENSED COMBINED
CONSOLIDATED STATEMENT OF OPERATIONS
FOR THE YEAR ENDED DECEMBER 31, 2021
(Amounts in thousands, except share and per share amounts)
| Year Ended | Year Ended | Year Ended | ||||||||||||||||
| December 31, 2021 | December 31, 2021 | December 31, 2021 | ||||||||||||||||
| Transaction | ||||||||||||||||||
| Avenue | Baergic | Accounting | Pro Forma | |||||||||||||||
| (Historical) | (Historical) | Adjustments | Notes | Combined | ||||||||||||||
| Expenses | ||||||||||||||||||
| Research and development | $ | 1,254 | $ | 342 | - | $ | 1,596 | |||||||||||
| General and administrative | 2,484 | 363 | 567 | 5(e) | 3,414 | |||||||||||||
| Total expenses | 3,738 | 705 | 567 | 5,010 | ||||||||||||||
| Operating loss | (3,738 | ) | (705 | ) | (567 | ) | (5,010 | ) | ||||||||||
| Other income (expense) | ||||||||||||||||||
| Interest income | 7 | - | - | 7 | ||||||||||||||
| Interest expense – related party | - | (307 | ) | - | (307 | ) | ||||||||||||
| Total other income (expense) | 7 | (307 | ) | - | (300 | ) | ||||||||||||
| Net loss | $ | (3,731 | ) | $ | (1,012 | ) | $ | (567 | ) | $ | (5,310 | ) | ||||||
| Basic and diluted net loss per share | $ | (3.29 | ) | $ | (1.21 | ) | ||||||||||||
| Basic and diluted weighted average shares outstanding | 1,133,170 | 4,380,648 | ||||||||||||||||
See accompanying notes to the unaudited pro forma condensed combined consolidated financial information.
Avenue basic and diluted weighted average shares outstanding and basic and diluted net loss per share are shown with the effect of one-for-fifteen reverse-split that took effect as of September 22, 2022.
56
UNAUDITED PRO FORMA CONDENSED COMBINED
CONSOLIDATED STATEMENT OF OPERATIONS
FOR THE YEAR ENDED DECEMBER 31, 2020
(Amounts in thousands, except share and per share amounts)
| Year Ended | Year Ended | Year Ended | ||||||||||||||||
| December 31, 2020 | December 31, 2020 | December 31, 2020 | ||||||||||||||||
| Transaction | ||||||||||||||||||
| Avenue | Baergic | Accounting | Pro Forma | |||||||||||||||
| (Historical) | (Historical) | Adjustments | Notes | Combined | ||||||||||||||
| Expenses | ||||||||||||||||||
| Research and development | $ | 2,866 | $ | 379 | - | $ | 3,245 | |||||||||||
| General and administrative | 2,347 | 360 | 567 | 5(e) | 3,274 | |||||||||||||
| Total expenses | 5,213 | 739 | 567 | 6,519 | ||||||||||||||
| Operating loss | (5,213 | ) | (739 | ) | (567 | ) | (6,519 | ) | ||||||||||
| Other income (expense) | ||||||||||||||||||
| Interest income | 62 | - | - | 62 | ||||||||||||||
| Interest expense – related party | - | (381 | ) | - | (381 | ) | ||||||||||||
| Total other income (expense) | 62 | (381 | ) | - | (319 | ) | ||||||||||||
| Net loss | $ | (5,151 | ) | $ | (1,120 | ) | $ | (567 | ) | $ | (6,838 | ) | ||||||
| Basic and diluted net loss per share | $ | (4.68 | ) | $ | (1.57 | ) | ||||||||||||
| Basic and diluted weighted average shares outstanding | 1,100,429 | 4,347,906 | ||||||||||||||||
See accompanying notes to the unaudited pro forma condensed combined consolidated financial information.
Avenue basic and diluted weighted average shares outstanding and basic and diluted net loss per share are shown with the effect of one-for-fifteen reverse-split that took effect as of September 22, 2022.
57
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
1. Description of the Merger
On May 11, 2022, Avenue entered into a stock contribution agreement (the “Contribution Agreement”) with Fortress, pursuant to which Fortress agreed to transfer its ownership of a majority of the outstanding shares (common and preferred) in a private subsidiary company of Fortress, Baergic Bio, Inc. (“Baergic Bio”), to Avenue. As of June 30, 2022, Fortress owns approximately 61% of Baergic’s common stock and 100% of Baergic’s Class A Preferred Stock. Under the Contribution Agreement, Fortress also agreed to assign to Avenue certain intercompany agreements existing between Fortress and Baergic, including a Founders Agreement and Management Services Agreement. Consummation of the transactions contemplated by the Contribution Agreement is subject to the satisfaction of certain conditions precedent, including: (i) the closing of an equity financing by Avenue resulting in gross proceeds of no less than $7.5 million, (ii) the agreement by InvaGen to (A) have 100% of its shares in Avenue repurchased by Avenue and (B) terminate certain of the agreements into which it entered with Avenue and/or Fortress in connection with InvaGen’s 2019 equity investment in Avenue, which will eliminate certain negative consent rights of InvaGen over Avenue and restore certain rights and privileges of Fortress in Avenue, and (iii) the sustained listing of Avenue’s Common Stock on Nasdaq. Avenue also entered into the Share Repurchase Agreement with InvaGen regarding the repurchase of the shares of its Common Stock it holds and the termination of the Historic Rights, although no assurance can be given that the other required consents and approvals for the closing of the Contribution Agreement will be obtained or that the closing conditions will be satisfied in a timely manner or at all.
2. Reverse Stock Split
On September 22, 2022, Avenue filed a Certificate of Amendment to its Third Amended and Restated Certificate of Incorporation (the “Amendment”) with the Secretary of State of the State of Delaware to (i) effect a one-for-fifteen reverse stock split (the “Reverse Stock Split”) of the Company’s shares of common stock, $0.0001 par value (the “Common Stock”), and (ii) effect a related reduction in the number of the Company’s authorized shares from 50,000,000 to 20,000,000 (the “Authorized Share Reduction”). All share and per share information has been retroactively adjusted to give effect to the reverse stock split for all periods presented, unless otherwise indicated.
As a result of the Reverse Stock Split, every fifteen shares of the Company’s pre-reverse split Common Stock were combined and reclassified as one share of Common Stock. Proportionate voting rights and other rights of common stockholders were not affected by the reverse split, other than as a result of the payment for fractional shares. No fractional shares were issued in connection with the Reverse Stock Split. Stockholders who would otherwise hold a fractional share of Common Stock received (upon surrender to the exchange agent of certificates representing such shares), a cash payment in lieu thereof, without interest or deduction, rounded to the nearest cent, in an amount equal to the product obtained by multiplying (a) the closing price per share of our common stock as reported on the Nasdaq Stock Market as of September 22, 2022, the effective date of the Reverse Stock Split, by (b) the fraction of one share owned by the stockholder. The total amount paid in consideration for the fractional shares was approximately $10,000.
Proportionate adjustments were made to the per share exercise price and/or the number of shares issuable upon the exercise or vesting of all restricted stock award/units and warrants outstanding at September 22, 2022, which resulted in a proportional decrease in the number of shares of the Company’s common stock reserved for issuance upon exercise or vesting of such restricted stock award/units and warrants, and, in the case of warrants, a proportional increase in the exercise price of all such stock options and warrants.
3. Basis of Presentation
The unaudited pro forma condensed combined financial information is prepared in accordance with Article 11 of SEC Regulation S-X.
The transaction will be accounted as a transaction between entities under common control such that Avenue will recognize the assets and liabilities of Baergic Bio received in the transaction at their historical carrying amounts, as reflected in the historical consolidated financial statements of Baergic Bio. No Goodwill or intangibles will be recognized.
The unaudited pro forma condensed combined consolidated balance sheet data gives effect to the transaction as if it had occurred on June 30, 2022. The unaudited pro forma condensed combined consolidated statements of operations data for the six months ended June 30, 2022 and the years ended December 31, 2021, and 2020 give effect to the transaction as if it had occurred on January 1, 2020.
The unaudited pro forma condensed combined financial information is presented solely for informational purposes and is not necessarily indicative of the combined results of operations or financial position that might have been achieved for the periods or dates indicated, nor is it necessarily indicative of the future results of the combined company. The unaudited pro forma condensed combined financial information has not been adjusted to give effect to certain expected financial benefits of the merger, such as tax savings, cost synergies or revenue synergies, or the anticipated costs to achieve these benefits, including the cost of integration activities. The unaudited pro forma condensed combined financial information does not reflect possible adjustments related to restructuring or integration activities that have yet to be determined. However, the impact of such transaction expenses is reflected in the unaudited pro forma combined balance sheet as a decrease to accumulated deficit and additional paid-in capital and as an increase to accrued expenses.
58
4. Accounting Policies
The unaudited pro forma condensed combined consolidated financial information has been compiled in a manner consistent with the accounting policies of Avenue. Following the common control transaction, the combined company will conduct a review of accounting policies of Baergic Bio in an effort to determine if differences in accounting policies require further reclassification of results of operations or reclassification of assets or liabilities to conform to Avenue’s accounting policies and classifications. As a result of that review, the combined company may identify differences among the accounting policies of the companies that, when conformed, could have a material impact on the unaudited pro forma condensed combined consolidated financial information.
5. Transaction Accounting Adjustments
Transaction Accounting Adjustments
The following provides explanations of the various adjustments to the unaudited pro forma condensed combined balance sheet:
| (a) | Represents the net proceeds from an assumed post June 30, 2022 Avenue issuance of $12.0 million of common stock (3,636,365 post-split units * $3.30 offering price). | |
| (b) | Represents underwriting fees of approximately $1.02 million (8.5% of $12.0 million) related to the offering. | |
| (c) | Represents payment of $3.0 million to InvaGen to repurchase 388,888 Company shares (after giving effect to the 1:15 reverse stock split), which shares are assumed to be cancelled at the time of repurchase. | |
| (d) | Represents eliminations through consolidation of accounts payable and accrued expenses – related party from assignment of Master Services Agreement from Fortress to Avenue for Baergic. | |
| (e) | Represents approximately $0.6 million of transaction costs expected to be incurred in connection with the transaction and the common stock issuance, of which none was incurred or accrued for on the balance sheet as of June 30, 2022. The adjustment was reflected in the balance sheet as a $0.6 million reduction in cash and a $0.6 million reduction in additional paid in capital. | |
| (f) | Represents the recording of the non-controlling interest of Baergic Bio.
As discussed in Note (a) to these unaudited pro forma condensed consolidated financial statements, Fortress is contributing to Avenue a controlling financial interest in Baergic Bio. As such, the combined company will consolidate Baergic Bio, but does not own 100% of the economic interest in Baergic Bio. The non-controlling interest in Baergic Bio, historically owned by entities other than Fortress and that will continue to be owned by entities other than Avenue, is 39%.
The non-controlling interest amount is calculated as the total stockholder’s equity (deficit) of Baergic times the non-controlling interest percentage of 39%. | |
| (g) | Represents equity adjustments due to transaction and the one-for-fifteen reverse stock split.
The Company’s board of directors approved a reverse split of shares of the Company’s common stock and convertible preferred stock on a one-for-fifteen basis (the “Reverse Stock Split”), which was effected on September 22, 2022. The par value and the number of authorized shares of the preferred stock and common stock were not adjusted in connection with the Reverse Stock Split. All references to common stock, preferred stock, share data, per share data and related information contained in the unaudited pro forma condensed combined consolidated financial information has been adjusted to reflect the effect of the Reverse Stock Split for all periods presented. No fractional shares of the Company’s common stock were issued in connection with the Reverse Stock Split. Any fractional share resulting from the Reverse Stock Split was rounded down to the nearest whole share, and any stockholder entitled to a fractional share as a result of the Reverse Stock Split received a cash payment in lieu of receiving fractional shares. | |
| (h) | Represents forgiveness of notes payable – related party and accrued interest – related party between Fortress and Baergic, which was agreed to by the companies on October 2, 2022. |
59
| i. | Adjustments to common stock as follows: |
| (in thousands) | Amount | |||
| Par value Avenue shares issued for cash | $ | - | ||
Effect of 1:15 reverse stock split | (3 | ) | ||
| Total pro forma adjustments | $ | (3 | ) | |
Effect of 1:15 reverse stock split based on estimated reduction in Avenue shares as of June 30, 2022 (22,134,784 issued and outstanding) times the par value ($0.0001 per share), plus new shares issued in the offering times the par value, less shares redeemed from InvaGen times the par value.
| ii. | Adjustments to paid-in capital as follows: |
| (in thousands) | Amount | |||
| Gross proceeds of common stock issuance | $ | 12,000 | ||
| Underwriting fees | (1,020 | ) | ||
| Repurchase and cancellation of shares | (3,000 | ) | ||
| Elimination through consolidation of accounts payable and accrued expenses – related party | 1,270 | |||
| Transaction costs related to offering | (567 | ) | ||
| Recording of Baergic Bio non-controlling interest | 2,369 | |||
| Effect of 1:15 reverse stock split | 3 | |||
| Forgiveness of note payable – related party and accrued interest – related party | 4,796 | |||
| Total pro forma adjustments | $ | 15,851 | ||
| iii. | Adjustments to non-controlling interest as follows: |
| (in thousands) | Amount | |||
| Recording of Baergic Bio non-controlling interest | $ | 2,369 | ||
| Total pro forma adjustments | $ | 2,369 | ||
6. Loss per Share
The unaudited pro forma weighted average number of basic and diluted shares outstanding for the six months ended June 30, 2022 and for the years ended December 31, 2021, and 2020 is calculated as follows:
| Six Months Ended June 30, 2022 | ||||
| Pro forma net loss | $ | (4,569 | ) | |
| Pro forma weighted average shares outstanding - basic and diluted | 4,676,759 | |||
| Net loss per share - basic and diluted | $ | (0.98 | ) | |
| Year Ended December 31, 2021 | ||||
| Pro forma net loss | $ | (5,310 | ) | |
| Pro forma weighted average shares outstanding - basic and diluted | 4,380,648 | |||
| Net loss per share - basic and diluted | $ | (1.21 | ) | |
| Year Ended December 31, 2020 | ||||
| Pro forma net loss | $ | (6,838 | ) | |
| Pro forma weighted average shares outstanding - basic and diluted | 4,347,906 | |||
| Net loss per share - basic and diluted | $ | (1.57 | ) | |
| Pro Forma Weighted Average Shares | ||||
| Avenue shareholders - as of June 30, 2022 | 1,429,282 | |||
| Avenue shareholders - equity issuance | 3,636,365 | |||
| Repurchased and cancelled shares | (388,888 | ) | ||
| Pro forma weighted average shares outstanding, basic and diluted | 4,676,759 | |||
Pro forma weight average shares outstanding shown for the periods ending June 30, 2022, December 31, 2021, and December 31, 2020, include the effect of the one-for-fifteen reverse stock split, equity issuance of 3,636,365 shares, and repurchase and cancelled shares of 388,888 shares.
Pro Forma Weighted Average Shares for Avenue shareholders – as of June 30, 2022 also include the effect of the one-for-fifteen reverse stock split.
60
Overview
As previously disclosed, on May 11, 2022, we entered into a Contribution Agreement with Fortress pursuant to which Fortress agreed to transfer its ownership of a majority of the outstanding shares (common and preferred) in a private subsidiary company of Fortress, Baergic Bio, to the Company. Under the Contribution Agreement, Fortress also agreed to assign to the Company certain intercompany agreements existing between Fortress and Baergic, including a Founders Agreement and Management Services Agreement. Consummation of the transactions contemplated by the Contribution Agreement is subject to the satisfaction of certain conditions precedent, including, inter alia: (i) the closing of an equity financing by the Company resulting in gross proceeds of no less than $7.5 million, (ii) the agreement by InvaGen to (A) have 100% of its shares in the Company repurchased by the Company and (B) terminate certain of the agreements into which it entered with the Company and/or Fortress in connection with InvaGen’s 2019 equity investment in the Company, which will eliminate certain negative consent rights of InvaGen over the Company and restore certain rights and privileges of Fortress in the Company, and (iii) our Common Stock then being listed and trading on Nasdaq without any pending action that would terminate such listing. As previously disclosed, we have since entered into the Share Repurchase Agreement with InvaGen regarding the repurchase of the shares of our Common Stock it holds and the termination of the Historic Rights and the right to nominate three members of our board of directors. We also expect that our reverse stock split, effective as of September 22, 2022, and the proceeds raised from this offering will result in Nasdaq determining that we are again compliant with its rules. However, no assurance can be given that the other required consents and approvals for the closing of the Contribution Agreement will be obtained or that the closing conditions will be satisfied in a timely manner or at all.
Baergic Bio is a clinical-stage pharmaceutical company founded in December 2019 that focuses on the development of pharmaceutical products for the treatment of disorders associated with the central nervous systems (CNS). Its pipeline currently consists of a single compound, BAER-101, a selective GABA-A α2 and α3 positive allosteric modulator (“PAM”). We plan to take advantage of BAER-101’s unique selectivity profile to develop it in areas of unmet need, namely epilepsy and acute anxiety disorders.
Description of BAER-101 (formerly known as AZD7325)
Modulators of GABA-A receptors (GABA-ARs) have entered a new age in their clinical development with multiple assets moving forward since the 2019 U.S. FDA approval of brexanolone (Zulresso®). These compounds are being developed for a host of therapeutic indications including epilepsy, anxiety, pain, depression, and other disease states. BAER-101 is a small molecule potentiator of GABA-ARs with oral bioavailability that preferentially activates α2- and α3-containing GABA-ARs. As such, BAER-101 is one of four other non-steroidal GABA-AR potentiators in clinical development with selectivity to individual receptor subtypes:
| · | darigabat – α2/3/5-preferring (Phase 2) for epilepsy and panic disorder being developed by Cerevel Therapeutics (Nasdaq:CERE) |
| · | KRM-II-81 – α2/3-preferring (Preclinical) being developed by RespireRx (OTCQB:RSPI) |
| · | SAN711, α3-preferring (Phase 1) for migraine and pain being developed by Saniona (OMX:SANION) |
| · | ENX101, α2/3/5-preferring (Phase 1b) for epilepsy being developed by Engrail Therapeutics (Private) |
Preclinical data have substantiated the efficacy of BAER-101 as a novel anxiolytic and antiepileptic with potential for also treating Fragile X Syndrome. Consistent with its selectivity over α1-preferring GABA-ARs, BAER-101 may have a reduced propensity to produce sedation and memory impairment.
61
BAER-101 has demonstrated efficacy in several preclinical models that may predict efficacy in patients. BAER-101 produced potent anxiolytic-like effects in rodents, anticonvulsant activity in certain rodent seizure models, efficacy in rodent models of Dravet syndrome and in a rodent model of Fragile X syndrome. Studies in rodents have also demonstrated good tolerability, with minimal ability to induce motor and memory impairment, characteristic effects of non α-selective GABA-AR potentiators like the BDZ diazepam. EEG power analysis also differentiated BAER-101 from compounds like the BDZ lorazepam. Physical dependence and abuse liability of BAER-101 are also reduced in model systems compared to non-selective GABA-AR modulators.
Diseases Currently Treated with Nonselective GABA-A Drugs: Benzodiazepines
Epilepsy Background
Epilepsy is a chronic disease that manifests as recurrent unprovoked seizures from abnormal electrical discharge in the brain. An epilepsy diagnosis requires at least 2 unprovoked seizures.
The current standard of care treatment involves use of one or more anti-epileptic drugs (AED). Side effects of approved therapies include dizziness, nausea, headache, vomiting, fatigue, vertigo, ataxia, blurred vision, and tremor. Even with the availability of approved drugs, 30% of patients do not achieve seizure control with two or more AEDs and these patients are characterized as drug-resistant. The consequences of poorly controlled epilepsy can be quite severe and include shortened lifespan, excessive bodily injury, neuropsychological and psychiatric impairment, and social disability.
Benzodiazepines are a class of AED that are used to treat seizures (convulsions). The use of benzodiazepines for a chronic disease such as epilepsy is limited by the side effect profile including drowsiness, confusion, dizziness, impaired coordination, increased risk of falls and accidents, and depression. More serious side effects include memory problems and behavioral changes — such as increased risk taking, delirium, and risk of dependence.
Studies have shown that people with seizures have a deficit in GABA neurotransmission. GABA, a major inhibitory neurotransmitter, inhibits the activity of nerves that could initiate the seizure. Benzodiazepines mainly work by affecting the gamma amino-butyric acid (GABA) neurotransmitters in the brain. Specifically, benzodiazepines enhance the activity of GABA by binding to its receptor, and opening its chloride channel, enabling release of GABA, resulting in anticonvulsant activity.
Benzodiazepines act non-selectively by enhancing the inhibitory effects of gamma-amino butyric acid (GABA) at GABA-A receptors containing either an α1, α2, α3, or α5 subunit. The field has progressed with the development of selective GABA-A receptor modulators that preferentially target one or more receptor subunits and BAER-101 is such a modulator. BAER-101 is selective for the α2, α3 receptor subunits an, as a result we believe it should provide an anti-convulsant effect while limiting the side effects associated with the α1 receptor.
Acute Anxiety Background
Panic disorder is a common form of an acute anxiety disorder manifesting as frequent panic attacks unrelated to specific situations. Panic attacks involve sudden, intense episodes of apprehension, terror, feelings of impending doom and intense urge to flee, with symptoms reaching peak intensity within 10 minutes. Patients can end up presenting to the emergency room simulating physical symptoms which can include labored breathing, heart palpitations, nausea, upset stomach, chest pain, feelings of choking and smothering, dizziness, sweating, lightheadedness, chills, heat sensations, and trembling. Other symptoms may include depersonalization, derealization, and fears of mental illness, losing control, or dying.
Panic disorder is treated with a combination of cognitive behavioral therapy and anxiolytics (drugs that reduce anxiety). These drugs include the following classes: benzodiazepines, tricyclics, selective serotonin reuptake inhibitors (SSRIs), and serotonin-norepinephrine reuptake inhibitors (SNRIs). Side effects can be problematic with existing medications especially with benzodiazepines, that have the potential for symptom exacerbation and abuse.
62
BAER-101 is a selective GABA-A α2 and α3 PAM that offers a potential new treatment for patients with epilepsy and acute anxiety
Key features of BAER-101 result from its selective effect on α2 and α3 containing GABA-A receptors (see below), resulting in efficacy in animal models of efficacy, anxiety, and Fragile X, without the side effect profile consistent with non-selective GABA-A compounds, such as BDZ. Some of these predictions have been confirmed in clinical trials.
1. BAER-101 in vitro demonstrates a selective mechanism of action:
α2 and α3 containing GABA-A receptor selectivity
In vitro pharmacology of BAER-101 displays high affinity interaction with GABA-ARs containing α1, α2, or α3 subunits and much lower affinity for α5-containing GABA-ARs (Table 1). Despite interacting with α1, α2 and α3, in functional assays, BAER-101 selectively potentiates α2 and α3 containing GABA-ARs significantly more than those containing α1 (Table 2).
Table 1. Binding of BAER-101 (AZD7325) at different GABA-AR populations.
| Receptor subtype | Potency, Ki mean ±SD (nM) | |
| GABAAα1 | 0.5 ±0.3 | |
| GABAAα2 | 0.3 ±0.2 | |
| GABAAα3 | 1.3 ±0.9 | |
| GABAAα5 | 230 ±65 |
Table 2. Enhancement of GABA-AR function by BAER-101 (AZD7325) when applied at different concentrations to an EC10 concentration of GABA
| BAER-101 (AZD7325) Concentration (nM) | ||||||||||
| Subtype | Functional measure | 1 | 10 | 100 | 1000 | |||||
| GABAAα1 | % Potentiation ±SDa | 7.5 ±4.0 | 7.8 ±3.8 | 7.4 ±7.2 | 10.9 ±7.2 | |||||
| % Relative potentiationb | 4.1 | 4.3 | 4 | 6 | ||||||
| GABAAα2 | % Potentiation ±SDa | 5.0 ±3.4 | 20.4 ±4.8 | 42.9 ±8.5 | 53.0 ±10.4 | |||||
| % Relative potentiationb | 1.7 | 7 | 14.7 | 18.2 | ||||||
| GABAAα3 | % Potentiation ±SDa | 2.4 ±1.4 | 7.1 ±4.7 | 44.5 ±11.9 | 56.4 ±4.8 | |||||
| % Relative potentiationb | 0.6 | 1.9 | 12.1 | 15.4 | ||||||
| GABAAα5 | % Potentiation ±SDa | -0.1 ±4.9 | -1.1 ±8.1 | 3.4 ±8.2 | 18.9 ±12.3 | |||||
| % Relative potentiationb | (0.1) | (0.5) | 1.5 | 8.4 | ||||||
a % Potentiation is the percentage increase in baseline GABA current upon co-application of BAER-101 (AZD7325).
b % Relative potentiation was calculated from the ratio of % Potentiation at 1µM to maximal diazepam response (set at 100%) and expressed as a percentage.
63
2. BAER-101 (AZD7325) efficacy in relevant pre-clinical models
a. Anti-Convulsant Effects
Pilot studies were carried out with mice to establish the anticonvulsant potential of BAER-101. In these studies (n=4), mice were dosed with BAER-101 and then given a convulsant stimulus after 0.25, 0.5, 1, 2, or 4 h post dosing. Mice were given BAER-101 by the intraperitoneal (i.p.) route at 10 mg/kg and by the oral (p.o.) route at 30 mg/kg. The following convulsant stimuli were assessed: maximal electroshock, pentylenetetrazol, and 6Hz corneal stimulation. BAER-101 reduced convulsions by 33% in the maximal electroshock test in one experiment, by 25% in the 6Hz assay, and 75% in the pentylenetetrazol test. There was sedation at 30 mg/kg in some mice in only one of the studies conducted.
In a mouse model of Dravet syndrome using Scn1a+/- mice, Nomura et al. (2019) showed that BAER-101 (AZD7325) was protective against seizures without notable sedation.
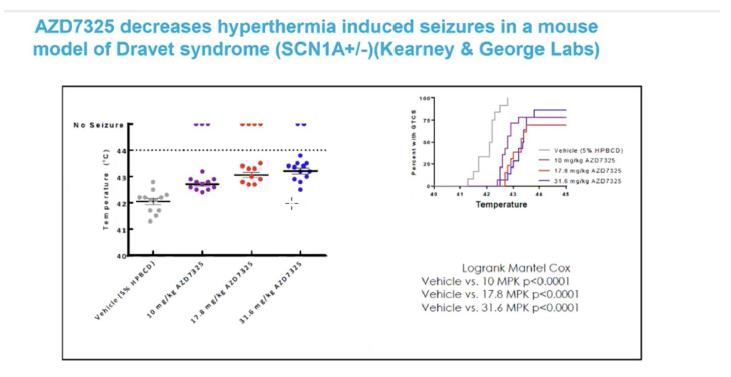
b. Anxiolytic Effects
BAER-101 (AZD7325) was tested in three different rodent models to determine anxiolytic efficacy: the punished responding model (PR) the rat fear potentiated startle (FPS) model, and the elevated maze model (EM).
In the PR model, BAER-101 (AZD7325) increased the rate of punished responding at all doses tested greater than a threshold dose, demonstrating) compared to their vehicle controls. Acute anxiolytic activity was similar to or greater than that of the reference BDZ diazepam (~250% at 3.5 μmol/kg, po).
Similarly, in the FPS, BAER-101 was demonstrated to have an anxiolytic effect similar to BDZ.
BAER-101 (AZD7325) was also tested in the FPS rodent model for anxiety. The significant difference between the response in the vehicle group and the BAER-101 (AZD7325) treated group in the light suggests that the 10.6 mg/kg dose has produced an anxiolytic effect.
In the EM model, BAER-101 (AZD7325) increased the percent time spent on the open arms at all 3 of the doses tested compared to vehicle-treated animals, with the magnitude of effect the same across all 3 doses.
c. Effects in a Genetic Model of Fragile X Syndrome (FXS)
Fragile X Syndrome is one of the most common causes of inherited intellectual disability, and is often accompanied by other symptoms, including behavioral challenges and seizures. Fragile X Mental Retardation Protein (FMRP) is functionally lost in FXS. Schaefer et al. (2021) interrogated the potential protective effects of BAER-101 (AZD7325) in mice without this protein. BAER-101 (AZD7325) reduced hyperexcitability in cortical circuits, partially corrected the increased frequency-specific baseline cortical EEG power, reduced susceptibility to audiogenic seizures, and improved novel object memory. Although other behaviors in these mice were not improved by BAER-101 (AZD7325) (increased hippocampal dendritic spine density, open field activity, and marble burying), the primary cortical damping effects were viewed as therapeutically meaningful.
64
3. BAER-101 (AZD7325) reduces in vivo side effect profile in animal models
The in vitro profile (detailed above) translates to a non-sedative anxiolytic profile in vivo, as characterized in multiple rat models of sedation and anxiety. Non-clinical studies in rat and primate models of cognition and abuse liability demonstrate that BAER-101 (AZD7325) has a reduced side effect profile in these domains as well when compared to benzodiazepines. The safety profile of BAER-101 (AZD7325) results in robust margins between predicted maximum clinical exposures for efficacy versus the exposures noted to cause toxicity in the most sensitive species.
| a. | Sedation: |
For example, in multiple studies, observations in rats documented a lack of sedation or impairment of motor activity when BAER-101 (AZD7325) was given at doses that produce anxiolytic or anticonvulsant effects.
This differentiation was conformed when comparing the effects on EEG of BAER-101 with more sedating GABA-A compounds such as zolpidem and alprazolam (Fig. 1) with BAER-101 having minimal impact on any of the EEG bands representing brain activity.
65
Figure 1. Differentiation of effects on the EEG power spectrum of BAER-101 (AZD7325) from the characteristic sedation signatures of reference drugs, lorazepam and zolpidem in rats.
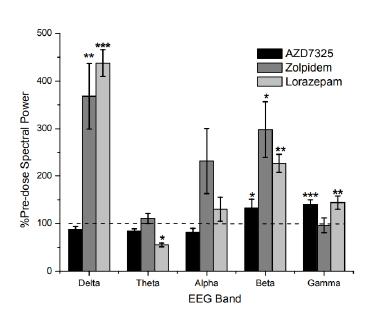
Functional impact of treatment with GABA-A modulators was measured in the Irwin test in mice to access multiple possible ‘side-effects’ of BAER-101 when given in vivo compared to diazepam. Diazepam produced greater motor impairment and for longer duration than BAER-101 (AZD7325).
| a. | Cognitive function: |
When tested in rats in a test of working memory, diazepam had marked effects on working memory even at doses as low as 1 mg/kg. BAER-101 (AZD7325) did not significantly impact working memory until the highest dose tested.
4. Translation of preclinical studies into human clinical results
In human trials, BAER-101 (AZD7325) has demonstrated favorable pharmacology consistent with the aforementioned animal studies, including high brain receptor occupancy, a good safety margin, and activity in patients with Generalized Anxiety Disorder (GAD) (although did not achieve statistical significance on the primary endpoint). Studies on epilepsy in humans have not yet been performed.
66
5. Extensive clinical trials
A total of 722 male and female subjects have been exposed to BAER-101 (AZD7325) in clinical trials and the drug has an established safety profile across multiple clinical studies. Studies completed to date include a single ascending dose (SAD) study, a multiple ascending dose (MAD) study, a Japanese SAD study, a [11C]flumazenil-labeled PET study, an exploratory study specifically designed to address cognition and sedation, a study to evaluate drug abuse potential, a study exploring BAER-101 (AZD7325)’s cytochrome P450 (CYP) induction potential, a study investigating the co-administration of BAER-101 (AZD7325) with an oral contraceptive (OC), and two Phase 2 efficacy studies in patients with generalized anxiety disorder (GAD), all performed by AstraZeneca. BAER-101 (AZD7325) has been administered as a single dose up to 100 mg and repeated doses up to 50 mg administered once daily (QD) for 7 days or 15 mg twice daily (BID) for 28 days. Cincinnati Children’s Hospital Medical Center has also completed an investigator-initiated pilot trial in patients with Fragile X Syndrome.
Clinical Development Plan
Post-acquisition, we expect to advance BAER-101 development for epilepsies and acute anxiety/panic disorder with the initiation of Phase 1b trials in 2023. We plan to test BAER-101 in a photosensitivity model of epilepsy. This model includes study participants who have reproducible generalized epileptiform discharges on electroencephalogram (EEG) stimulated by flashing lights within a range of frequencies call a photoparoxysmal response (PPR). This study population translates well into future epilepsy population studies. In addition, we plan to test BAER-101 in a hypercapnia CO2 inhalation challenge study. Hypercapnia results in increased fear and panic as measured by the Visual Analogue Scales (VAS) and the Panic Symptom List (PSL). This is a translational model providing proof-of-principle for anxiolytic activity in clinical development.
67
DESCRIPTION OF SECURITIES TO BE REGISTERED
Avenue Therapeutics has one class of securities registered under Section 12 of the Securities Act of 1934, as amended: our Common Stock. The following description of our Common Stock is a summary and is qualified in its entirety by reference to our Third Amended and Restated Certificate of Incorporation, as amended, and our Amended and Restated By-Laws (the “By-Laws”), which are included as exhibits to the registration statement on Form S-1 of which this prospectus forms a part. We encourage you to read the Certificate of Incorporation and By-Laws as well as the applicable provisions of the General Corporation Law of the State of Delaware, as amended (the “DGCL”), for more information.
Authorized Capital Stock
Our authorized capital stock consists of 20,000,000 shares of Common Stock, with $0.0001 par value, and 2,000,000 shares of Preferred Stock, with $0.0001 par value, of which 250,000 have been designated as Class A Preferred Stock and the remainder of which are undesignated Preferred Stock.
As of March 21, 2022, there were 21,732,284 shares of our Common Stock outstanding held by 38 record stockholders, prior to the effect of the one-for-fifteen reverse stock split.
As of September 23, 2022, the date following the effective date of the one-for-fifteen reverse stock split, there were 1,475,652 shares of our Common Stock outstanding held by approximately 38 record stockholders. In addition, the authorized capital stock of Common Stock was reduced from 50,000,000 to 20,000,000.
Common Stock
Voting Rights
Holders of our Common Stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders and do not have cumulative voting rights. An election of directors by our stockholders shall be determined by a plurality of the votes cast by the stockholders entitled to vote on the election. Holders of Common Stock are entitled to receive proportionately any dividends as may be declared by our Board of Directors, subject to any preferential dividend rights of outstanding preferred stock.
Liquidation and Other Rights
In the event of our liquidation or dissolution, the holders of Common Stock are entitled to receive proportionately all assets available for distribution to stockholders after the payment of all debts and other liabilities and subject to the prior rights of any outstanding preferred stock. Holders of Common Stock have no preemptive, subscription, redemption or conversion rights. The rights, preferences and privileges of holders of Common Stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock that we may designate and issue in the future.
Listing
Our Common Stock is traded on the Nasdaq Capital Market under the symbol “ATXI.” The transfer agent and registrar for our Common Stock is VStock Transfer, LLC.
Dividends
Holders of Common Stock are entitled to receive proportionately any dividends as may be declared by our board of directors, subject to any preferential dividend rights of outstanding preferred stock. Pursuant to the certificate of designation relating to the series A preferred stock, we are prohibited from paying dividends on our Common Stock until all dividends required to be paid to the holders of our Class A Preferred Stock have been paid or declared and set apart for payment.
68
Anti-Takeover Effects of Various Provisions of Delaware Law and Avenue Therapeutics’ Certificate of Incorporation and By-Laws
Provisions of the DGCL and our Certificate of Incorporation and By-Laws could make it more difficult to acquire Avenue Therapeutics by means of a tender offer, a proxy contest or otherwise, or to remove incumbent officers and directors. These provisions, including those summarized below, may encourage certain types of coercive takeover practices and takeover bids.
Delaware Anti-Takeover Statute. In general, Section 203 of the DGCL prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years following the time the person became an interested stockholder, unless the business combination or the acquisition of shares that resulted in a stockholder becoming an interested stockholder is approved in a prescribed manner. Generally, a “business combination” includes a merger, asset or stock sale or other transaction resulting in a financial benefit to the interested stockholder. Generally, an “interested stockholder” is a person who, together with affiliates and associates, owns (or within three years prior to the determination of interested stockholder status did own) 15% or more of a corporation’s voting stock. However, our Certificate of Incorporation provides that we are not subject to the anti-takeover provisions of Section 203 of the DGCL.
Removal. Subject to the rights of any holders of any outstanding series of our Preferred Stock, stockholders may remove our directors with or without cause. Removal will require the affirmative vote of holders of a majority of our voting stock.
Size of Board and Vacancies. Our By-Laws provide that the number of directors be fixed exclusively by the board of directors. Any vacancies created on its board of directors resulting from any increase in the authorized number of directors or the death, resignation, retirement, disqualification, removal from office or other cause will be filled by a majority of the board of directors then in office, even if less than a quorum is present, or by a sole remaining director. Any director appointed to fill a vacancy on our board of directors will be appointed until the next annual meeting and until his or her successor has been elected and qualified.
Requirements for Advance Notification of Stockholder Nominations and Proposals. Our By-Laws establish advance notice procedures with respect to stockholder proposals and nomination of candidates for election as directors other than nominations made by or at the direction of its board of directors or a committee of our board of directors.
Undesignated Preferred Stock. Our board of directors is authorized to issue up to 2,000,000 shares of preferred stock without additional stockholder approval, which preferred stock could have voting rights or conversion rights that, if exercised, could adversely affect the voting power of the holders of Common Stock. The issuance of shares of preferred stock may have the effect of delaying, deferring or preventing a change in control of the Company without any action by the Company’s stockholders.
Limitation on Liability of Directors and Indemnification of Directors and Officers
Elimination of Liability of Directors. The DGCL authorizes corporations to limit or eliminate the personal liability of directors to corporations and their stockholders for monetary damages for breaches of directors’ fiduciary duties as directors, and our Certificate of Incorporation includes such an exculpation provision. Our Certificate of Incorporation provides that, to the fullest extent permitted by the DGCL, no director will be personally liable to us or to our stockholders for monetary damages for breach of fiduciary duty as a director. While our Certificate of Incorporation provides directors with protection from awards for monetary damages for breaches of their duty of care, it does not eliminate this duty. Accordingly, our Certificate of Incorporation has no effect on the availability of equitable remedies such as an injunction or rescission based on a director’s breach of his or her duty of care. The provisions apply to an officer of Avenue Therapeutics only if he or she is a director of Avenue Therapeutics and is acting in his or her capacity as director, and do not apply to officers of Avenue Therapeutics who are not directors. Additionally, our Certificate of Incorporation provides that, to the fullest extent permitted by law, we renounce any interest or expectancy in a transaction or matter that may be a corporate opportunity for us if it was presented to, or acquired, created or developed by, or which otherwise comes into the possession of, (i) any director on our board of directors who is not an employee of the Company or any of its subsidiaries, or (ii) any holder of our Class A Preferred Stock or any affiliate or other related person of any such holder, other than someone who is an employee of the Company or any of its subsidiaries, and no person shall have any duty to present such corporate opportunity to us and will not be liable to us for pursuing or acquiring such opportunity, or referring such opportunity to a third party.
69
Indemnification of Directors, Officers and Employees. Our By-Laws require us to indemnify any person who was or is a party or is threatened to be made a party to, or was otherwise involved in, a legal proceeding by reason of the fact that he or she is or was a director, officer or employee of Avenue Therapeutics or, while a director, officer or employee of Avenue Therapeutics, is or was serving at our request in a fiduciary capacity with another enterprise (including any corporation, partnership, limited liability company, joint venture, trust, association or other unincorporated organization or other entity and any employee benefit plan, to the fullest extent authorized by the DGCL, as it exists or may be amended, against all expense, liability and loss (including attorneys’ fees, judgments, fines, U.S. Employee Retirement Income Security Act of 1974, as amended, excise taxes or penalties and amounts paid in settlement by or on behalf of such person) actually and reasonably incurred in connection with such service. We are authorized under our By-Laws to carry directors’ and officers’ insurance protecting us, any director, officer or employee of ours or, against any expense, liability or loss, whether or not we have the power to indemnify the person under the DGCL. We may, to the extent authorized from time to time, indemnify any of our agents to the fullest extent permitted with respect to directors, officers and employees in our By-Laws.
The limitation of liability and indemnification provisions in our Certificate of Incorporation and By-Laws may discourage stockholders from bringing a lawsuit against our directors for breach of fiduciary duty. These provisions also may reduce the likelihood of derivative litigation against our directors and officers, even though such an action, if successful, might otherwise benefit us and our stockholders. By its terms, the indemnification provided for in our By-Laws is not exclusive of any other rights that the indemnified party may be or become entitled to under any law, agreement, vote of stockholders or directors, provisions of our Certificate of Incorporation or By-Laws or otherwise. Any amendment, alteration or repeal of our By-Laws’ indemnification provisions is, by the terms of our By-Laws, prospective only and will not adversely affect the rights of any indemnity in effect at the time of any act or omission occurring prior to such amendment, alteration or repeal.
Warrants to be issued in this Offering
The following summary of certain terms and provisions of the warrants included in the units offered hereby is not complete and is subject to, and qualified in its entirety by the provisions of the form of Warrant, which is filed as an exhibit to the registration statement of which this prospectus is a part. Prospective investors should carefully review the terms and provisions set forth in the form of Warrant.
Exercisability. The warrants are exercisable immediately and at any time up to the date that is five years after their original issuance. The warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice and, at any time a registration statement registering the offer and sale of the shares of Common Stock underlying the warrants under the Securities Act is effective and available for the issuance of such shares, or an exemption from registration under the Securities Act is available for the issuance of such shares, by payment in full in immediately available funds for the number of shares of Common Stock purchased upon such exercise. If a registration statement registering the offer and sale of the shares of Common Stock underlying the warrants under the Securities Act is not effective or available and an exemption from registration under the Securities Act is not available for the issuance of such shares, the holder may elect to exercise the warrant through a cashless exercise, in which case the holder would receive upon such exercise the net number of shares of Common Stock determined according to the formula set forth in the warrant. No fractional shares of Common Stock will be issued in connection with the exercise of a warrant. In lieu of fractional shares, we will pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price.
Exercise Limitation. A holder will not have the right to exercise any portion of the warrant if the holder (together with its affiliates and certain related parties) would beneficially own in excess of 4.99% of the number of shares of our Common Stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the warrants. However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99%, provided that any increase in such percentage shall not be effective until 61 days following notice from the holder to us.
70
Exercise Price. The exercise price per whole share of Common Stock purchasable upon exercise of the warrants is equal to $3.30 (100% of the public offering price per unit). The exercise price is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our Common Stock and also upon any distributions of assets, including cash, stock or other property to our stockholders.
Dilutive Issuance Adjustments. If, while the warrant is outstanding, we engage in any transaction involving the issue or sale of our shares of Common Stock or equivalent securities at an effective price per share less than the exercise price of the warrant then in effect (such lower price, the “Base Share Price”), the exercise price of the warrant shall be reduced to equal the Base Share Price. There shall only be one such adjustment to the exercise price, if any, while the warrant is outstanding.
Transferability. Subject to applicable laws, the warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange Listing. We do not intend to list the warrants on any securities exchange or nationally recognized trading system.
Warrant Agent. The warrants will be issued in registered form under a warrant agency agreement between VStock Transfer, LLC, as warrant agent, and us. The warrants will initially be represented only by one or more global warrants deposited with the warrant agent, as custodian on behalf of The Depository Trust Company (DTC) and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC.
Fundamental Transactions. In the event of a fundamental transaction, as described in the warrants and generally including any reorganization, recapitalization or reclassification of our Common Stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding Common Stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding Common Stock, the holders of the warrants will be entitled to receive upon exercise of the warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the warrants immediately prior to such fundamental transaction.
Rights as a Stockholder. Except as otherwise provided in the warrants or by virtue of such holder’s ownership of shares of our Common Stock, the holder of a warrant does not have the rights or privileges of a holder of our Common Stock, including any voting rights, until the holder exercises the warrant.
Governing Law. The warrants and the warrant agency agreement are governed by New York law.
Pre-funded Warrants to be issued in this Offering
The following summary of certain terms and provisions of the pre-funded warrants included in the pre-funded units offered hereby is not complete and is subject to, and qualified in its entirety by the provisions of the form of pre-funded warrant, which is filed as an exhibit to the registration statement of which this prospectus is a part. Prospective investors should carefully review the terms and provisions set forth in the form of pre-funded warrant.
Exercisability. The pre-funded warrants are exercisable immediately and may be exercised at any time until the pre-funded warrants are exercised in full. The pre-funded warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice and, at any time a registration statement registering the offer and sale of the shares of Common Stock underlying the pre-funded warrants under the Securities Act is effective and available for the issuance of such shares, or an exemption from registration under the Securities Act is available for the issuance of such shares, by payment in full in immediately available funds for the number of shares of Common Stock purchased upon such exercise. If a registration statement registering the offer and sale of the shares of Common Stock underlying the pre-funded warrants under the Securities Act is not effective or available and an exemption from registration under the Securities Act is not available for the issuance of such shares, the holder may elect to exercise the pre-funded warrants through a cashless exercise, in which case the holder would receive upon such exercise the net number of shares of Common Stock determined according to the formula set forth in the warrant. No fractional shares of Common Stock will be issued in connection with the exercise of a pre-funded warrants. In lieu of fractional shares, we will pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price.
71
Exercise Limitation. A holder will not have the right to exercise any portion of the pre-funded warrant if the holder (together with its affiliates and certain related parties) would beneficially own in excess of 4.99% of the number of shares of our Common Stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the pre-funded warrants. However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99%, provided that any increase in such percentage shall not be effective until 61 days following notice from the holder to us.
Exercise Price. The exercise price per whole share of Common Stock purchasable upon exercise of the pre-funded warrants is $0.0001. The exercise price is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our Common Stock and also upon any distributions of assets, including cash, stock or other property to our stockholders.
Transferability. Subject to applicable laws, the pre-funded warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange Listing. We do not intend to list the pre-funded warrants on any securities exchange or nationally recognized trading system.
Warrant Agent. The pre-funded warrants will be issued in registered form under a warrant agency agreement between VStock Transfer, LLC, as warrant agent, and us. The pre-funded warrants will initially be represented only by one or more global warrants deposited with the warrant agent, as custodian on behalf of The Depository Trust Company (DTC) and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC.
Fundamental Transactions. In the event of a fundamental transaction, as described in the pre-funded warrants and generally including any reorganization, recapitalization or reclassification of our Common Stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding Common Stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding Common Stock, the holders of the pre-funded warrants will be entitled to receive upon exercise of the pre-funded warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the pre-funded warrants immediately prior to such fundamental transaction.
Rights as a Stockholder. Except as otherwise provided in the pre-funded warrants or by virtue of such holder’s ownership of shares of our Common Stock, the holder of a pre-funded warrant does not have the rights or privileges of a holder of our Common Stock, including any voting rights, until the holder exercises the pre-funded warrant.
Governing Law. The pre-funded warrants and the warrant agency agreement are governed by New York law.
72
MATERIAL UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The following is a discussion of certain material U.S. federal income tax consequences of the acquisition, ownership and disposition of our shares of common units (each consisting of one share of our common stock and one warrant to purchase one share of our common stock) and our pre-funded units (each consisting of one pre-funded warrant to purchase one share of our common stock and one warrant to purchase one share of our common stock), which we refer to as our securities, that are purchased in this offering by U.S. Holders (as defined below) and Non-U.S. Holders (as defined below). Because the components of a common unit and a pre-funded unit are generally separable at the option of the holder, the holder of a common unit or pre-funded unit generally should be treated, for U.S. federal income tax purposes, as the owner of the underlying share of our common stock and one warrant to purchase one share of our common stock in the case of a common unit and one pre-funded warrant and one warrant to purchase one share of our common stock in the case of a pre-funded unit. As a result, the discussion below with respect to holders of shares of our common stock, pre-funded warrants and warrants should also apply to holders of common units or pre-funded units (as the deemed owners of the underlying common stock, pre-funded warrants and warrants that constitute the units).
This discussion applies only to securities that are held as capital assets for U.S. federal income tax purposes and is applicable only to initial holders who are receiving our securities in this offering.
This discussion is a summary only and does not describe all of the tax consequences that may be relevant to you in light of your particular circumstances, including but not limited to the alternative minimum tax, the Medicare tax on certain investment income and the different consequences that may apply if you are subject to special rules that apply to certain types of investors (such as the effects of Section 451 of the federal income tax code (the “Code”), including but not limited to:
| · | bank and other financial institutions or financial services entities; |
| · | broker-dealers; |
| · | mutual funds; |
| · | retirement plans, individual retirement accounts or other tax-deferred accounts; |
| · | governments or agencies or instrumentalities thereof; |
| · | regulated investment companies; |
| · | pension plans; |
| · | “controlled foreign corporations,” “passive foreign investment companies,” “qualified foreign pension funds,” and corporations that accumulate earnings to avoid U.S. federal income tax; |
| · | real estate investment trusts; |
| · | expatriates or former long-term residents of the United States; |
| · | persons that actually or constructively own five percent or more of our voting shares; |
| · | insurance companies; |
| · | taxpayers subject to a mark-to-market method of accounting rules; |
| · | persons holding the securities as part of a “straddle,” constructive sale, hedge, conversion or other integrated or similar transaction; |
| · | U.S. holders (as defined below) whose functional currency is not the U.S. dollar; |
| · | persons subject to alternative minimum tax; |
| · | partnerships or other pass-through entities for U.S. federal income tax purposes and any beneficial owners of such entities; |
| · | tax-exempt entities; and |
| · | persons that acquired our securities pursuant to an exercise of employee share options, in connection with employee share incentive plans or otherwise as compensation or in connection with services. |
73
This discussion is based on the Code, and administrative pronouncements, judicial decisions and final, temporary and proposed Treasury regulations as of the date hereof, which are subject to change, possibly on a retroactive basis, and changes to any of which subsequent to the date of this prospectus may affect the tax consequences described herein. This discussion does not address any aspect of state, local or non-U.S. taxation, or any U.S. federal taxes (e.g., gift and estate taxes) other than income taxes.
We have not sought, and will not seek, a ruling from the IRS as to any U.S. federal income tax consequence described herein. The IRS may disagree with the discussion herein, and its determination may be upheld by a court. Moreover, there can be no assurance that future legislation, regulations, administrative rulings or court decisions will not adversely affect the accuracy of the statements in this discussion. You are urged to consult your tax advisor with respect to the application of U.S. federal tax laws to your particular situation, as well as any tax consequences arising under the laws of any state, local or foreign jurisdiction.
This discussion does not consider the tax treatment of partnerships or other pass-through entities or persons who hold our securities through such entities. If a partnership (or other entity or arrangement classified as a partnership or other pass-through entity for United States federal income tax purposes) is the beneficial owner of our securities, the United States federal income tax treatment of a partner or member in the partnership or other pass-through entity generally will depend on the status of the partner or member and the activities of the partnership or other pass-through entity. If you are a partner or member of a partnership or other pass-through entity holding our securities, we urge you to consult your own tax advisor.
THIS DISCUSSION IS ONLY A SUMMARY OF CERTAIN UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS ASSOCIATED WITH THE ACQUISITION, OWNERSHIP AND DISPOSITION OF OUR SECURITIES. EACH PROSPECTIVE INVESTOR IN OUR SECURITIES IS URGED TO CONSULT ITS OWN TAX ADVISOR WITH RESPECT TO THE PARTICULAR TAX CONSEQUENCES TO SUCH INVESTOR OF THE ACQUISITION, OWNERSHIP AND DISPOSITION OF OUR SECURITIES, INCLUDING THE APPLICABILITY AND EFFECT OF ANY UNITED STATES FEDERAL NON-INCOME, STATE, LOCAL, AND NON-U.S. TAX LAWS.
Allocation of Purchase Price and Characterization of a Unit
No statutory, administrative or judicial authority directly addresses the treatment of a unit or instruments similar to a unit for U.S. federal income tax purposes, and therefore, that treatment is not entirely clear. The acquisition of a common unit or pre-funded unit should be treated for U.S. federal income tax purposes as the acquisition of one share of our common stock and one warrant in the case of a common unit and one pre-funded warrant and one warrant in the case of a pre-funded unit, and we intend to treat the acquisition of a unit in this manner. For U.S. federal income tax purposes, each holder of a unit must allocate the purchase price paid by such holder for such unit among the underlying securities based on the relative fair market value of each at the time of issuance. Under U.S. federal income tax law, each investor must make its own determination of such value based on all the relevant facts and circumstances. Therefore, we strongly urge each investor to consult its tax advisor regarding the determination of value for these purposes. The price allocated to each share of our common stock, warrants and/or pre-funded warrants should constitute the holder’s initial tax basis in such share, warrant and/or pre-funded warrant, respectively. Any disposition of a Unit should be treated for U.S. federal income tax purposes as a disposition of the share of our common stock and warrant or pre-funded warrant and warrant comprising the Unit, and the amount realized on the disposition should be allocated among the underlying securities based on their respective relative fair market values at the time of disposition.
The foregoing treatment of the securities and a holder’s purchase price allocation are not binding on the IRS or the courts. Because there are no authorities that directly address instruments that are similar to the units, no assurance can be given that the IRS or the courts will agree with the characterization described above or the discussion below. Accordingly, each prospective investor is urged to consult its tax advisor regarding the tax consequences of an investment in a unit (including alternative characterizations of a unit). The balance of this discussion assumes that the characterization of the units described above is respected for U.S. federal income tax purposes.
U.S. Holders
This section applies to you if you are a “U.S. holder.” A U.S. holder is a beneficial owner of our shares of Common Stock who or that is, for U.S. federal income tax purposes:
| · | an individual who is a citizen or resident of the United States; |
| · | a corporation (or other entity taxable as a corporation) that is created or organized (or treated as created or organized) in or under the laws of the United States, any state thereof or the District of Columbia; or |
| · | an estate the income of which is subject to U.S. federal income tax regardless of its source; or |
| · | a trust, if (i) a court within the United States is able to exercise primary supervision over the administration of the trust and one or more U.S. persons (as defined in the Code) have authority to control all substantial decisions of the trust or (ii) it has a valid election in effect under Treasury Regulations to be treated as a U.S. person. |
Taxation of Distributions. If we pay distributions in cash or other property (other than certain distributions of our stock or rights to acquire our stock) to U.S. holders of shares of our Common Stock, such distributions generally will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Distributions in excess of current and accumulated earnings and profits will constitute a return of capital that will first be applied against and reduce (but not below zero) the U.S. holder’s adjusted tax basis in our Common Stock. Any remaining excess will be treated as gain realized on the sale or other disposition of the Common Stock and will be treated as described under “U.S. Holders — Gain or Loss on Sale, Taxable Exchange or Other Taxable Disposition of Common Stock” below.
74
Dividends we pay to a U.S. holder that is a taxable corporation generally will qualify for the dividends received deduction if the requisite holding period is satisfied. With certain exceptions (including, but not limited to, dividends treated as investment income for purposes of investment interest deduction limitations), and provided certain holding period requirements are met, dividends we pay to a non-corporate U.S. holder may constitute “qualified dividends” that will be subject to tax at the maximum tax rate accorded to long-term capital gains. If the holding period requirements are not satisfied, then a corporation may not be able to qualify for the dividends received deduction and would have taxable income equal to the entire dividend amount, and non-corporate holders may be subject to tax on such dividend at regular ordinary income tax rates instead of the preferential rate that applies to qualified dividend income.
Gain or Loss on Sale, Taxable Exchange or Other Taxable Disposition of our Securities. Upon a sale or other taxable disposition of our shares of Common Stock, warrants or pre-funded warrants, a U.S. holder generally will recognize capital gain or loss in an amount equal to the difference between the amount realized and the U.S. holder’s adjusted tax basis in such shares of Common Stock, warrants or pre-funded warrants. Any such capital gain or loss generally will be long-term capital gain or loss if the U.S. holder’s holding period for the Common Stock, warrants or pre-funded warrants so disposed of exceeds one year. If the holding period requirements are not satisfied, any gain on a sale or taxable disposition of our securities would be subject to short-term capital gain treatment and would be taxed at regular ordinary income tax rates. Long-term capital gains recognized by non-corporate U.S. holders will be eligible to be taxed at reduced rates. The deductibility of capital losses is subject to limitations.
Generally, the amount of gain or loss recognized by a U.S. holder is an amount equal to the difference between (i) the sum of the amount of cash and the fair market value of any property received in such disposition and (ii) the U.S. holder’s adjusted tax basis in its shares of Common Stock, warrants or pre-funded warrants disposed. A U.S. holder’s adjusted tax basis in its shares of Common Stock, warrants or pre-funded warrants generally will equal the U.S. holder’s acquisition cost (that is, the portion of the purchase price of a Unit allocated to a share of our common stock, warrant or pre-funded warrant, as described above under “— Allocation of Purchase Price and Characterization of a Unit”) reduced, in the case of a share of Common Stock, by any prior distributions treated as a return of capital. .
Information Reporting and Backup Withholding. In general, information reporting requirements may apply to dividends paid to a U.S. holder and to the proceeds of the sale or other disposition of our securities, unless the U.S. holder is an exempt recipient. Backup withholding may apply to such payments if the U.S. holder fails to provide a taxpayer identification number, a certification of exempt status or has been notified by the IRS that it is subject to backup withholding (and such notification has not been withdrawn).
Any amounts withheld under the backup withholding rules generally should be allowed as a refund or a credit against a U.S. holder’s U.S. federal income tax liability provided the required information is timely furnished to the IRS.
Non-U.S. Holders
This section applies to you if you are a “Non-U.S. holder.” As used herein, the term “Non-U.S. holder” means a beneficial owner of our common units or pre-funded units who is not a U.S. Holder or any other person that is for U.S. federal income tax purposes:
| · | a non-resident alien individual (other than certain former citizens and residents of the U.S. subject to U.S. tax as expatriates), |
| · | a foreign corporation, or |
| · | an estate or trust that is not a U.S. holder. |
The term “Non-U.S. Holder” generally does not include a U.S. Holder or a partnership or other entity classified as a partnership for U.S. federal income tax purposes and does not include an individual who is present in the United States for 183 days or more in the taxable year of disposition of the securities. If you are such an individual, you should consult your tax advisor regarding the U.S. federal income tax consequences of the acquisition, ownership or sale or other disposition of our securities.
75
Taxation of Distributions. In general, any distributions we make to a Non-U.S. holder of shares of our Common Stock, to the extent paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles), will constitute dividends for U.S. federal income tax purposes and, provided such dividends are not effectively connected with the Non-U.S. holder’s conduct of a trade or business within the United States, we will be required to withhold tax from the gross amount of the dividend at a rate of 30%, unless such Non-U.S. holder is eligible for a reduced rate of withholding tax under an applicable income tax treaty and provides proper certification of its eligibility for such reduced rate (usually on an IRS Form W-8BEN or W-8BEN-E). Any distribution not constituting a dividend will be treated first as reducing (but not below zero) the Non-U.S. holder’s adjusted tax basis in its shares of our Common Stock and, to the extent such distribution exceeds the Non-U.S. holder’s adjusted tax basis, as gain realized from the sale or other disposition of the Common Stock, which will be treated as described under “Non-U.S. Holders — Gain on Sale, Taxable Exchange or Other Taxable Disposition of Our Securities” below. If we are unable to determine, at a time reasonably close to the date of payment of a distribution on our Common Stock, what portion, if any, of the distribution will constitute a dividend, then we may withhold U.S. federal income tax on the basis of assuming that the full amount of the distribution will be a dividend. If we or another withholding agent apply over-withholding, a non-U.S. holder may be entitled to a refund or credit of any excess tax withheld by timely filing an appropriate claim with the IRS. In addition, if we determine that we are or are likely to be classified as a “United States real property holding corporation” (see “Non-U.S. Holders — Gain on Sale, Taxable Exchange or Other Taxable Disposition of Our Securities” below), we will withhold 15% of any distribution that exceeds our current and accumulated earnings and profits, including a distribution in redemption of shares of our Common Stock.
The withholding tax does not apply to dividends paid to a Non-U.S. holder who provides a Form W-8ECI, certifying that the dividends are effectively connected with the Non-U.S. holder’s conduct of a trade or business within the United States. Instead, the effectively connected dividends will be subject to regular U.S. income tax as if the Non-U.S. holder were a U.S. resident, subject to an applicable income tax treaty providing otherwise. A Non-U.S. corporation receiving effectively connected dividends may also be subject to an additional “branch profits tax” imposed at a rate of 30% (or a lower treaty rate).
Any documentation provided to an applicable withholding agent may need to be updated in certain circumstances. The certification requirements described above also may require a non-U.S. holder to provide its U.S. taxpayer identification number.
Gain on Sale, Taxable Exchange or Other Taxable Disposition of Common Stock. A Non-U.S. holder generally will not be subject to U.S. federal income or withholding tax in respect of gain recognized on a sale, taxable exchange or other taxable disposition of our Common Stock, warrants or pre-funded warrants, in each case without regard to whether such securities were held as part of a unit, unless:
| · | the gain is effectively connected with the conduct of a trade or business by the Non-U.S. holder within the United States (and, under certain income tax treaties, is attributable to a United States permanent establishment or fixed base maintained by the Non-U.S. holder); |
| · | the non-U.S. holder is a nonresident alien individual who is present in the United States for a period or periods aggregating 183 days or more in the taxable year of the disposition and certain other conditions are met, in which case the non-U.S. holder will be subject to a 30% tax (or such lower rate as may be specified by an applicable income tax treaty) on the amount by which the non-U.S. holder’s capital gains allocable to U.S. sources exceed capital losses allocable to U.S. sources during the taxable year of the disposition (without taking into account any capital loss carryovers); or |
| · | we are or have been a “U.S. real property holding corporation” for U.S. federal income tax purposes at any time during the shorter of the five-year period ending on the date of disposition or the period that the Non-U.S. holder held our Common Stock, and, in the case where shares of our Common Stock are regularly traded on an established securities market, the Non-U.S. holder has owned, directly or constructively, more than 5% of our Common Stock at any time within the shorter of the five-year period preceding the disposition or such Non-U.S. holder’s holding period for the shares of our Common Stock. There can be no assurance that our Common Stock will be treated as regularly traded on an established securities market for this purpose. Generally, a corporation is a U.S. real property holding corporation if the fair market value of its U.S. real property interests, as defined in the Code and applicable U.S. Treasury Regulations, equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other assets used or held for use in a trade or business. Although there can be no assurance, we do not believe that we are, or have been, a U.S. real property holding corporation for U.S. federal income tax purposes, or that we are likely to become one in the future. These rules may be modified for Non-U.S. Holders of warrants or pre-funded warrants. If we are or have been a “United States real property holding corporation” and you own warrants or pre-funded warrants, you are urged to consult your own tax advisor regarding the application of these rules. |
76
Unless an applicable treaty provides otherwise, gain described in the first bullet point above will be subject to tax at generally applicable U.S. federal income tax rates as if the Non-U.S. holder were a U.S. resident. Any gains described in the first bullet point above of a Non-U.S. holder that is a foreign corporation may also be subject to an additional “branch profits tax” at a 30% rate (or lower treaty rate).
If the third bullet point above applies to a Non-U.S. holder, gain recognized by such holder on the sale, exchange or other disposition of our Common Stock, warrants or pre-funded warrants, will generally be subject to tax at applicable U.S. federal income tax rates as if the Non-U.S. Holder were a U.S. resident. In addition, a buyer of our Common Stock, warrants or pre-funded warrants from any such holder may be required to withhold U.S. income tax at a rate of 15% of the amount realized upon such disposition if our Common Stock is not treated as regularly traded on an established securities market. We cannot determine whether we will be a United States real property holding corporation in the future. In general, we would be classified as a United States real property holding corporation if the fair market value of our “United States real property interests” equals or exceeds 50% of the sum of the fair market value of our worldwide real property interests plus our other assets used or held for use in a trade or business, as determined for U.S. federal income tax purposes.
Information Reporting and Backup Withholding. Information returns will be filed with the IRS in connection with payments of dividends and the proceeds from a sale or other disposition of our shares of Common Stock, warrants or pre-funded warrants. A Non-U.S. holder may have to comply with certification procedures to establish that it is not a United States person in order to avoid information reporting and backup withholding requirements. The certification procedures required to claim a reduced rate of withholding under a treaty will satisfy the certification requirements necessary to avoid the backup withholding as well. The amount of any backup withholding from a payment to a Non-U.S. holder will be allowed as a credit against such holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
FATCA Withholding Taxes. Provisions commonly referred to as “FATCA” impose withholding of 30% on payments of dividends (including constructive dividends) on our Common Stock to “foreign financial institutions” (which is broadly defined for this purpose and in general includes investment vehicles) and certain other Non-U.S. entities unless various U.S. information reporting and due diligence requirements (generally relating to ownership by U.S. persons of interests in or accounts with those entities) have been satisfied by, or an exemption applies to, the payee (typically certified as to by the delivery of a properly completed IRS Form W-8BEN-E). If FATCA withholding is imposed, a beneficial owner that is not a foreign financial institution will be entitled to a refund of any amounts withheld by filing a U.S. federal income tax return (which may entail significant administrative burden). Foreign financial institutions located in jurisdictions that have an intergovernmental agreement with the United States governing FATCA may be subject to different rules. Under certain circumstances, a Non-U.S. holder might be eligible for refunds or credits of such withholding taxes, and a Non-U.S. holder might be required to file a U.S. federal income tax return to claim such refunds or credits. Prospective investors should consult their tax advisers regarding the effects of FATCA on their investment in our securities.
The preceding discussion of material U.S. federal tax considerations is for general information only. It is not tax advice. You should consult your own tax advisors regarding the particular U.S. federal, state, local and non-U.S. tax consequences of purchasing, holding and disposing of our Common Stock, including the consequences of any proposed changes in applicable laws.
77
We will enter into an underwriting agreement with Aegis Capital Corp. as the sole underwriter (“Aegis” or the “Underwriter”), with respect to the units and pre-funded units being offered. Aegis is the sole book-running manager for the offering. Subject to the terms and conditions of an underwriting agreement between us and Aegis, we have agreed to sell to Aegis at the public offering price less the underwriting discounts set forth on the cover page of this prospectus, the number of shares of units listed next to its name in the following table:
| Name of Underwriter | Number
of Units | Number
of Pre-funded Units | |||||
| Aegis Capital Corp. | |||||||
| 2,652,065 | 984,300 | ||||||
| Total | 2,652,065 | 984,300 | |||||
The Underwriter is committed to purchase all the units or pre-funded units offered by this prospectus if they purchase any units. The Underwriter is not obligated to purchase the units covered by the Underwriter’s over-allotment option described below. The Underwriter is offering the units and pre-funded units, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, and other conditions contained in the underwriting agreement, such as the receipt by the Underwriter of officer’s certificates and legal opinions. The Underwriter reserves the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Over-Allotment Option
We have granted to the underwriter an option, exercisable no later than 45 calendar days after the date of the closing of the offering to purchase up to an additional 545,454 additional shares of Common Stock, additional pre-funded warrants or additional warrants from us, in any combination thereof, representing 15% of the securities sold in the offering.
Discounts and Commissions; Expenses
The following table shows the public offering price, underwriting discount and proceeds, before expenses, to us. The information assumes either no exercise or full exercise by Aegis of the over-allotment option.
| Per Unit | Total Without Over- Allotment Option | Total With Full Over- Allotment Option | ||||||||||
| Public offering price | $ | 3.30 | $ | 3.2999 | $ | 12,000,004.50 | ||||||
| Underwriting discount (8.5%) | $ | 0.2805 | $ | 0.2805 | $ | 1,020,000.38 | ||||||
| Proceeds, before expenses, to us | $ | 3.0195 | $ | 3.0194 | $ | 10,980,004.12 | ||||||
The underwriting discount will be 8.5% for the offering and a non-accountable expense allowance of equal to 1.0% of the offering. In addition, we will pay $125,000 for fees and expenses including “road show,” diligence and reasonable legal fees and disbursements for the underwriter’s counsel. We estimate that total expenses payable by us in connection with this offering, other than the underwriting discount, will be approximately $567,199.
Lock-Up Agreements
Pursuant to certain “lock-up” agreements, our executive officers, employees, directors and stockholders holding at least ten percent (10%) of our outstanding shares of common stock have agreed, subject to certain exceptions, not to offer, sell, assign, transfer, pledge, contract to sell, or otherwise dispose of or announce the intention to otherwise dispose of, or enter into any swap, hedge or similar agreement or arrangement that transfers, in whole or in part, the economic risk of ownership of, directly or indirectly, engage in any short selling of any common stock or securities convertible into or exchangeable or exercisable for any common stock, whether currently owned or subsequently acquired, without the prior written consent of the Underwriter, for a period of 90 days from the date of effectiveness of this offering. In addition, each such person agrees that, without the prior written consent of the Underwriter, such person will not, during the restricted period, make any demand for, or exercise any right with respect to, the registration of the resale of any shares of common stock or any security convertible into or exercisable or exchangeable for common stock.
78
Company Standstill
For a period of twelve (12) months from the closing date of the offering, we have agreed that without the prior written consent of Aegis, we will not (a) offer, sell, issue, or otherwise transfer or dispose of, directly or indirectly, any equity of the Company or any securities convertible into or exercisable or exchangeable for equity of the Company; (b) file or caused to be filed any registration statement with the Commission relating to the offering of any equity of the Company or any securities convertible into or exercisable or exchangeable for equity of the Company; or (c) enter into any agreement or announce the intention to effect any of the actions described in subsections (a) or (b) hereof (all of such matters, the “Standstill”). As long as none of such equity securities shall be saleable in the public market until the expiration of the twelve (12) month period described above, the following matters shall not be prohibited by the Standstill: (i) the adoption of an equity incentive plan and the grant of awards or equity pursuant to any equity incentive plan, and the filing of a registration statement on Form S-8; (ii) the issuance of equity securities in connection with an acquisition or a strategic relationship, which may include the sale of equity securities; and (iii) other customary exceptions as may be agreed to in the Underwriting Agreement. In no event should any equity transaction during the Standstill period result in the sale of equity at an offering price to the public less than that of the Offering referred herein.
Indemnification
We have agreed to indemnify the Underwriter against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the Underwriter may be required to make for these liabilities.
Price Stabilization, Short Positions, and Penalty Bids
In connection with this offering, the Underwriter may engage in transactions that stabilize, maintain or otherwise affect the price of our common stock. Specifically, the Underwriter may over-allot in connection with this offering by selling more shares than are set forth on the cover page of this prospectus. This creates a short position in our common stock for its own account. The short position may be either a covered short position or a naked short position. In a covered short position, the number of shares of common stock over-allotted by the Underwriter is not greater than the number of shares of common stock that it may purchase in the over-allotment option. In a naked short position, the number of shares of common stock involved is greater than the number of shares of common stock in the over-allotment option. To close out a short position, the Underwriter may elect to exercise all or part of the over-allotment option. The Underwriter may also elect to stabilize the price of our common stock or reduce any short position by bidding for, and purchasing, common stock in the open market.
The Underwriter may also impose a penalty bid. This occurs when a particular underwriter or dealer repays selling concessions allowed to it for distributing a security in this offering because the underwriter repurchases that security in stabilizing or short covering transactions.
Finally, the Underwriter may bid for, and purchase, shares of our common stock in market making transactions, including “passive” market making transactions as described below.
These activities may stabilize or maintain the market price of our common stock at a price that is higher than the price that might otherwise exist in the absence of these activities. The Underwriter is not required to engage in these activities, and may discontinue any of these activities at any time without notice. These transactions may be effected on Nasdaq, in the over-the-counter market, or otherwise.
79
In connection with this offering, the Underwriter, or its affiliates may engage in passive market making transactions in our common stock immediately prior to the commencement of sales in this offering, in accordance with Rule 103 of Regulation M under the Exchange Act. Rule 103 generally provides that:
| · | a passive market maker may not effect transactions or display bids for our common stock in excess of the highest independent bid price by persons who are not passive market makers; |
| · | net purchases by a passive market maker on each day are generally limited to 30% of the passive market maker’s average daily trading volume in our common stock during a specified two-month prior period or 200 shares, whichever is greater, and must be discontinued when that limit is reached; and |
| · | passive market making bids must be identified as such. |
Electronic Distribution
A prospectus in electronic format may be made available on a website maintained by the Underwriter. The Underwriter may agree to allocate a number of units for sale to their online brokerage account holders. Internet distributions will be allocated by the Underwriter that may make Internet distributions on the same basis as other allocations. In connection with the offering, the Underwriter or syndicate members may distribute prospectuses electronically. No forms of electronic prospectus other than prospectuses that are printable as Adobe® PDF will be used in connection with this offering.
The Underwriter has informed us that they do not expect to confirm sales of shares offered by this prospectus to accounts over which they exercise discretionary authority.
Other than the prospectus in electronic format, the information on any Underwriter’s website and any information contained in any other website maintained by the Underwriter is not part of the prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or any underwriter in its capacity as underwriter and should not be relied upon by investors.
McGuireWoods LLP, Charlotte, North Carolina, will pass upon the validity of the securities we are offering by this prospectus. The underwriter is being represented in connection with this offering by Kaufman & Canoles, P.C., Richmond, Virginia.
The financial statements of Avenue Therapeutics, Inc. as of December 31, 2021 and 2020 and for each of the two years in the period ended December 31, 2021 incorporated by reference in this prospectus and in the Registration Statement have been so incorporated in reliance on the report of BDO USA, LLP, an independent registered public accounting firm, incorporated herein by reference, given on the authority of said firm as experts in auditing and accounting. The report on the financial statements contains an explanatory paragraph regarding the Company's ability to continue as a going concern.
The financial statements of Baergic Bio, Inc. as of December 31, 2021 and 2020 and for each of the years in the two-year period ended December 31, 2021, have been included herein and in the registration statement in reliance on the report of KPMG LLP, independent registered public accounting firm, appearing elsewhere herein, and upon the authority of said firm as experts in auditing and accounting. The audit report covering the December 31, 2021 financial statements contains an explanatory paragraph that states that the Company’s recurring losses from operations raise substantial doubt about the entity’s ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of that uncertainty.
80
WHERE YOU CAN FIND MORE INFORMATION
We file reports and proxy statements with the SEC. These filings include our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and proxy statements on Schedule 14A, as well as any amendments to those reports and proxy statements, which are available free of charge through our website as soon as reasonably practicable after we file them with, or furnish them to, the SEC. Our Internet website address is www.avenuetx.com. Our website and the information contained on, or that can be accessed through, the website will not be deemed to be incorporated by reference in, and are not considered part of, this prospectus. You should not rely on any such information in making your decision whether to purchase our securities. The SEC also maintains a website at www.sec.gov that contains reports, proxy and information statements and other information regarding us and other issuers that file electronically with the SEC.
We have filed with the SEC a registration statement on Form S-1 under the Securities Act relating to the securities being offered by this prospectus. This prospectus, which constitutes part of that registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules which are part of the registration statement. For further information about us and the securities offered, see the registration statement and the exhibits and schedules thereto. Statements contained in this prospectus regarding the contents of any contract or any other document to which reference is made are not necessarily complete, and, in each instance where a copy of a contract or other document has been filed as an exhibit to the registration statement, reference is made to the copy so filed, each of those statements being qualified in all respects by the reference.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” into this prospectus the information we file with the SEC in other documents, which means that we can disclose important information to you by referring you to those documents instead of having to repeat the information in this prospectus. The information incorporated by reference is considered to be part of this prospectus, and later information that we file with the SEC will automatically update and supersede such information. We incorporate by reference the documents listed below and any future information filed (rather than furnished) with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act between the date of this prospectus and the date all securities to which this prospectus relates have been sold or the offering is otherwise terminated and also between the date of the initial registration statement and prior to effectiveness of the registration statement, provided, however, that we are not incorporating any information furnished under Item 2.02 or Item 7.01 of any Current Report on Form 8-K:
| · | our Annual Report on Form 10-K for the year ended December 31, 2021, filed with the SEC on March 25, 2022; |
| · | our Quarterly Reports on Form 10-Q for the quarter ended March 31, 2022, filed with the SEC on May 16, 2022 and for the quarter ended June 30, 2022, filed with the SEC on August 15, 2022; and |
| · | our Current Reports on Form 8-K and Form 8-K/A filed with the SEC on January 7, 2022, February 11, 2022, February 16, 2022, March 8, 2022, March 31, 2022, March 30, 2022, April 5, 2022, May 13, 2022, May 16, 2022, May 25, 2022, July 29, 2022, August 3, 2022, August 12, 2022, August 31, 2022, September 22, 2022 and September 30, 2022. |
We will furnish without charge to you a copy of any or all of the documents incorporated by reference, including exhibits to these documents, upon written or oral request. Direct your written request to: Corporate Secretary, Avenue Therapeutics, Inc., 2 Gansevoort Street, 9th Floor, New York NY 10014, or (781) 652-4500.
A statement contained in a document incorporated by reference into this prospectus shall be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus, any prospectus supplement or in any other subsequently filed document which is also incorporated in this prospectus modifies or replaces such statement. Any statements so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
81
Financial Statements (audited)
December 31, 2021 and 2020
F-1
Independent Auditors’ Report
Board of Directors
Baergic Bio, Inc.
Report on the Audit of the Financial Statements
Opinion
We have audited the financial statements of Baergic Bio, Inc. (the Company), which comprise the balance sheets as of December 31, 2021 and 2020, and the related statements of operations, stockholders’ equity, and cash flows for the years then ended, and the related notes to the financial statements.
In our opinion, the accompanying financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020, and the results of its operations and its cash flows for the years then ended in accordance with U.S. generally accepted accounting principles.
Basis for Opinion
We conducted our audits in accordance with auditing standards generally accepted in the United States of America (GAAS). Our responsibilities under those standards are further described in the Auditors’ Responsibilities for the Audit of the Financial Statements section of our report. We are required to be independent of the Company and to meet our other ethical responsibilities, in accordance with the relevant ethical requirements relating to our audits. We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.
Substantial Doubt About the Entity’s Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has suffered recurring losses from operations, and has stated that substantial doubt exists about the Company's ability to continue as a going concern. Management's evaluation of the events and conditions and management's plans regarding these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. Our opinion is not modified with respect to this matter.
Responsibilities of Management for the Financial Statements
Management is responsible for the preparation and fair presentation of the financial statements in accordance with U.S. generally accepted accounting principles, and for the design, implementation, and maintenance of internal control relevant to the preparation and fair presentation of financial statements that are free from material misstatement, whether due to fraud or error.
In preparing the financial statements, management is required to evaluate whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the Company’s ability to continue as a going concern for one year after the date that the financial statements are available to be issued.
Auditors’ Responsibilities for the Audit of the Financial Statements
Our objectives are to obtain reasonable assurance about whether the financial statements as a whole are free from material misstatement, whether due to fraud or error, and to issue an auditors’ report that includes our opinion. Reasonable assurance is a high level of assurance but is not absolute assurance and therefore is not a guarantee that an audit conducted in accordance with GAAS will always detect a material misstatement when it exists. The risk of not detecting a material misstatement resulting from fraud is higher than for one resulting from error, as fraud may involve collusion, forgery, intentional omissions, misrepresentations, or the override of internal control. Misstatements are considered material if there is a substantial likelihood that, individually or in the aggregate, they would influence the judgment made by a reasonable user based on the financial statements.
F-2
In performing an audit in accordance with GAAS, we:
| ● | Exercise professional judgment and maintain professional skepticism throughout the audit. |
| ● | Identify and assess the risks of material misstatement of the financial statements, whether due to fraud or error, and design and perform audit procedures responsive to those risks. Such procedures include examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. |
| ● | Obtain an understanding of internal control relevant to the audit in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control. Accordingly, no such opinion is expressed. |
| ● | Evaluate the appropriateness of accounting policies used and the reasonableness of significant accounting estimates made by management, as well as evaluate the overall presentation of the financial statements. |
| ● | Conclude whether, in our judgment, there are conditions or events, considered in the aggregate, that raise substantial doubt about the Company’s ability to continue as a going concern for a reasonable period of time. |
We are required to communicate with those charged with governance regarding, among other matters, the planned scope and timing of the audit, significant audit findings, and certain internal control related matters that we identified during the audit.
| /s/ KPMG LLP |
New York, New York
August 31, 2022
F-3
BAERGIC BIO, INC.
BALANCE SHEETS
(in thousands, except share amounts)
| December 31, 2021 | December 31, 2020 | |||||||
| ASSETS | ||||||||
| Current Assets: | ||||||||
| Cash | $ | 10 | $ | 7 | ||||
| Total current assets | 10 | 7 | ||||||
| Total Assets | $ | 10 | $ | 7 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| Current Liabilities: | ||||||||
| Accounts payable and accrued expenses | 3 | $ | 3 | |||||
| Accounts payable and accrued expenses - related party | 1,020 | 520 | ||||||
| Accrued interest – related party | 564 | 257 | ||||||
| Notes payable – related party | 3,961 | 3,771 | ||||||
| Total current liabilities | 5,548 | 4,551 | ||||||
| Total Liabilities | 5,548 | 4,551 | ||||||
| Commitments and Contingencies | ||||||||
| Stockholders’ Deficit | ||||||||
| Preferred Stock ($0.0001 par value), 2,000,000 shares authorized and 250,000 shares outstanding as of December 31, 2021 and 2020 | — | — | ||||||
| Common Stock ($0.0001 par value), 50,000,000 shares authorized and 14,297,173 and 13,828,212 shares issued and outstanding as of December 31, 2021 and 2020, respectively | 1 | 1 | ||||||
| Additional paid-in capital | 140 | 122 | ||||||
| Accumulated deficit | (5,679 | ) | (4,667 | ) | ||||
| Total Stockholders’ Deficit | (5,538 | ) | (4,544 | ) | ||||
| Total Liabilities and Stockholders’ Deficit | $ | 10 | $ | 7 | ||||
See accompanying notes to financial statements.
F-4
BAERGIC BIO, INC.
STATEMENTS OF OPERATIONS
(in thousands)
| For the years ended | ||||||||
| December 31, | ||||||||
| 2021 | 2020 | |||||||
| Operating expenses: | ||||||||
| Research and development | $ | 342 | $ | 379 | ||||
| General and administrative | 363 | 360 | ||||||
| Total operating expenses | 705 | 739 | ||||||
| Loss from operations | (705 | ) | (739 | ) | ||||
| Other expense: | ||||||||
| Interest expense – related party | 307 | 381 | ||||||
| Total other expense | 307 | 381 | ||||||
| Net Loss | $ | (1,012 | ) | $ | (1,120 | ) | ||
See accompanying notes to financial statements.
F-5
BAERGIC BIO, INC.
STATEMENTS OF STOCKHOLDERS’ EQUITY
(in thousands, except share amounts)
| Additional | Total | |||||||||||||||||||||||||||
| Preferred Shares | Common Shares | Paid-in | Accumulated | Stockholders’ | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | Deficit | ||||||||||||||||||||||
| Balances at December 31, 2019 | 250,000 | $ | — | 12,242,192 | $ | 1 | $ | 89 | $ | (3,547 | ) | $ | (3,457 | ) | ||||||||||||||
| Stock-based compensation expense | — | — | 850,000 | — | 12 | — | 12 | |||||||||||||||||||||
| Issuance of common shares – License Agreement | — | — | 395,400 | — | 11 | — | 11 | |||||||||||||||||||||
| Issuance of common shares – Founders Agreement | — | — | 340,620 | — | 10 | — | 10 | |||||||||||||||||||||
| Net loss | — | — | — | — | — | (1,120 | ) | (1,120 | ) | |||||||||||||||||||
| Balances at December 31, 2020 | 250,000 | $ | — | 13,828,212 | $ | 1 | $ | 122 | $ | (4,667 | ) | $ | (4,544 | ) | ||||||||||||||
| Stock-based compensation expense | — | — | — | — | 4 | — | 4 | |||||||||||||||||||||
| Issuance of common shares – License Agreement | — | — | 117,006 | — | 4 | — | 4 | |||||||||||||||||||||
| Issuance of common shares – Founders Agreement | — | — | 351,955 | — | 10 | — | 10 | |||||||||||||||||||||
| Net loss | — | — | — | — | — | (1,012 | ) | (1,012 | ) | |||||||||||||||||||
| Balances at December 31, 2021 | 250,000 | $ | — | 14,297,173 | $ | 1 | $ | 140 | $ | (5,679 | ) | $ | (5,538 | ) | ||||||||||||||
See accompanying notes to financial statements.
F-6
BAERGIC BIO, INC.
STATEMENTS OF CASH FLOWS
(in thousands)
| For the years ended December 31, |
||||||||
| 2021 | 2020 | |||||||
| Cash Flows from Operating Activities: | ||||||||
| Net loss | $ | (1,012 | ) | $ | (1,120 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Interest expense | 307 | 381 | ||||||
| Stock-based compensation expense | 4 | 12 | ||||||
| Issuance of common shares – License Agreement | 4 | 11 | ||||||
| Issuance of common shares – Founders Agreement | 10 | 10 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts payable and accrued expenses | — | (181 | ) | |||||
| Accounts payable and accrued expenses – related parties | 500 | 500 | ||||||
| Net cash used in operating activities | (187 | ) | (387 | ) | ||||
| Cash Flows from Financing Activities: | ||||||||
| Proceeds from notes payable | 190 | 394 | ||||||
| Net cash provided by financing activities | 190 | 394 | ||||||
| Net increase in cash and cash equivalents | 3 | 7 | ||||||
| Cash and cash equivalents at beginning of period | 7 | — | ||||||
| Cash and cash equivalents at end of period | 10 | 7 | ||||||
See accompanying notes to financial statements.
F-7
Notes to Financial Statements
Note 1 – Organization and Description of Business
Baergic Bio, Inc. (the “Company” or “Baergic”) was incorporated in Delaware on June 10, 2015, however did not commence substantial operations until the execution of its license agreements in 2019. In December 2019, Baergic entered into two license agreements: (i) a License Agreement (the “AZ License”) with AstraZeneca AB (“AZ”) to acquire an exclusive license to patent and related intellectual property rights pertaining to their proprietary compound Gamma-aminobutyric acid receptor A alpha 2 & 3 (GABAA α2,3) positive allosteric modulators (collectively, the “AZ IP”); and (ii) an Exclusive License Agreement (the “Cincinnati License”) with Cincinnati Children’s Hospital Medical Center (“Cincinnati”) to acquire patent and related intellectual property rights pertaining to a GABA inhibitor program for neurological disorders (the “Cincinnati IP”). Baergic is a clinical-stage pharmaceutical company focused on the development of pharmaceutical products for the treatment of disorders associated with the central nervous system.
The Company is a majority-controlled subsidiary of Fortress Biotech, Inc. (“Fortress” or “Parent”).
Going Concern Considerations
Since inception, the Company has incurred operating losses and the Company’s operations have been financed primarily through an intercompany note from Fortress, on an as-needed basis. As of December 31, 2021, the Company’s stockholders’ deficit was $5.5 million. Further, the Company is not yet generating revenue and expects to continue to incur significant costs for the foreseeable future in pursuit of its development and financing plans and may never become profitable. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Note 2 – Significant Accounting Policies
Basis of Presentation
The Company’s financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”). The Company has no subsidiaries.
All intercompany transactions between Fortress and Baergic are classified as due from or due to related party in the financial statements.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Actual results could differ from those estimates.
Cash
The Company considers highly liquid investments with a maturity of three months or less when purchased to be cash equivalents. Cash at December 31, 2021 and 2020, consisted entirely of cash in institutions in the United States.
Research and Development Costs
Research and development costs are expensed as incurred. Advance payments for goods and services that will be used in future research and development activities are expensed when the activity has been performed or when the goods have been received rather than when the payment is made. Upfront and milestone payments due to third parties that perform research and development services on the Company’s behalf will be expensed as services are rendered or when the milestone is achieved.
F-8
Research and development costs primarily consist of personnel related expenses, including salaries, benefits, travel, and other related expenses, stock-based compensation, payments made to third parties for license and milestone costs related to in-licensed products and technology, payments made to third party contract research organizations for preclinical and clinical studies, investigative sites for clinical trials, consultants, the cost of acquiring and manufacturing clinical trial materials, costs associated with regulatory filings, laboratory costs and other supplies.
In accordance with Accounting Standards Codification (“ASC”) 730-10-25-1, Research and Development, costs incurred in obtaining licenses are charged to research and development expense if the rights licensed have not reached commercial feasibility and has no alternative future use. The licenses purchased by the Company require substantial completion of research and development, regulatory and marketing approval efforts to reach commercial feasibility and has no alternative future use. Accordingly, the total purchase price for the licenses acquired is reflected as research and development expense in the Company’s Statements of Operations (see Note 3).
Annual PIK Dividend to Class A Preferred Stockholders
The Company issued 250,000 shares of Class A Preferred Stock to Fortress. The Class A Preferred Stock entitle the holder to an annual stock dividend equal to 2.5% of the fully diluted outstanding equity of the Company (“PIK Dividend”, see Note 6). The PIK Dividend was part of the consideration payable for formation of the Company and the identification of certain assets, including the licenses contributed to Baergic by Fortress (see Note 3).
Pursuant to the Certificate of Incorporation, the Company issued 351,955 shares of common stock to Fortress for the PIK Dividend, representing 2.5% of the fully-diluted outstanding equity of Baergic on December 16, 2021 and is recorded in the Statement of Stockholders’ Equity at December 31, 2021, as Issuance of common shares – Founders Agreement. The Company recorded an expense of approximately $10,000 in research and development expense related to these issuable shares during the year ended December 31, 2021.
Pursuant to the Certificate of Incorporation, the Company issued 340,620 shares of common stock to Fortress for the PIK Dividend, representing 2.5% of the fully-diluted outstanding equity of Baergic on December 16, 2020 and is recorded in the Statement of Stockholders’ Equity at December 31, 2020, as Issuance of common shares – Founders Agreement. The Company recorded an expense of approximately $10,000 in research and development expense related to these issuable shares during the year ended December 31, 2020.
Fair Value Measurement
The Company follows accounting guidance on fair value measurements for financial assets and liabilities measured at fair value on a recurring basis. Under the accounting guidance, fair value is defined as an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or a liability.
The accounting guidance requires fair value measurements be classified and disclosed in one of the following three categories:
Level 1: Quoted prices in active markets for identical assets or liabilities.
Level 2: Observable inputs other than Level 1 prices, for similar assets or liabilities that are directly or indirectly observable in the marketplace.
Level 3: Unobservable inputs which are supported by little or no market activity and that are financial instruments whose values are determined using pricing models, discounted cash flow methodologies, or similar techniques, as well as instruments for which the determination of fair value requires significant judgment or estimation.
The fair value hierarchy also requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. Assets and liabilities measured at fair value are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. The Company’s assessment of the significance of a particular input to the fair value measurement in its entirety requires management to make judgments and consider factors specific to the asset or liability.
F-9
Stock-Based Compensation
The Company expenses stock-based compensation to employees and directors over the requisite service period based on the estimated grant-date fair value of the awards and forfeitures, which are recorded upon occurrence. Restricted stock awards and restricted stock unit awards are expensed under the straight-line method over the vesting period. Expense for awards with performance-based vesting criteria will be measured and recorded if and when it becomes probable that the performance criteria will be achieved.
Income Taxes
The Company records income taxes using the asset and liability method. Deferred income tax assets and liabilities are recognized for the future tax effects attributable to temporary differences between the financial statement carrying amounts of existing assets and liabilities and their respective income tax bases, and operating loss and tax credit carryforwards. The Company establishes a valuation allowance if management believes it is more likely than not that the deferred tax assets will not be recovered based on an evaluation of objective verifiable evidence. For tax positions that are more likely than not of being sustained upon audit, the Company recognizes the largest amount of the benefit that is greater than 50% likely of being realized. For tax positions that are not more likely than not of being sustained upon audit, the Company does not recognize any portion of the benefit.
Comprehensive Loss
The Company has no components of other comprehensive loss, and therefore, comprehensive loss equals net loss.
Recently Issued Accounting Pronouncements
In August 2020, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2020-06, Debt-Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging-Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity, which simplifies accounting for convertible instruments by removing major separation models required under current GAAP. The ASU removes certain settlement conditions that are required for equity contracts to qualify for the derivative scope exception and it also simplifies the diluted earnings per share calculation in certain areas. This guidance is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2023. Early adoption will be permitted. The Company is currently evaluating the impact of this standard on its financial statements.
F-10
Note 3 – License, Clinical Trial and Sponsored Research Agreements
License Agreements
AstraZeneca AB License Agreement
Pursuant to the terms of the AZ License, Baergic paid an upfront fee of $3.0 million and issued 2,492,192 common shares equal to 19.95% of Baergic to AZ as consideration for AZ License. In connection with the issuance of the shares, Baergic also provided AZ with anti-dilution protection until the earliest to occur of (i) receipt of $75 million in aggregate gross proceeds from the sale of new securities to third-party investors, (ii) such time as AZ holds fewer than 25% of shares issued in connection with the execution of the license agreement, (iii) closing of a change of control transaction, or (iv) immediately following the consummation of an IPO of Baergic. Shares issuable under the anti-dilution provisions are being accounted for as share-based payment transactions issued as part of the acquisition of the license.
Baergic valued the stock grant to AZ utilizing a Required Rate of Return model to determine the weighted market value of invested capital, discounted by a lack of marketability of 44.6%, weighted average cost of capital of 20.5%, and net of debt utilized, and an option pricing model using a risk-free rate of 1.69%, a maturity of 5.0 years, and a volatility of 84% resulting in a value of $0.029 per share or $0.1 million on December 31, 2019.
In addition, if Baergic issues shares of any class or series of Capital Stock that is senior to Common Stock, including any Class A Preferred Stock, then the shares of the Common Stock then held by AZ shall be convertible, at AZ’s sole option, into shares of such class or series of Capital Stock (“Exchange Right”). Such conversion shall require no additional consideration from AZ and will be into a number of shares of such class or series of Capital Stock to maintain AZ’s fully-diluted ownership percentage. The Exchange Right will terminate upon the earlier of (i) such time that AZ and its affiliates collectively hold fewer than 25% of the shares of Capital Stock issued to AZ in connection with the execution of the transaction, as adjusted for any stock combination, stock split, stock dividend, recapitalization or other similar transaction; (ii) the closing of a Complete Change of Control (a deemed liquidation event or an outside entity becoming the owner of 100% of the total voting power of the equity securities of Baergic then-outstanding that are entitle to vote); and (iii) immediately before the consummation of an IPO of Baergic.
Development milestone payments totaling approximately $75 million in the aggregate are due upon achievement of each milestone. Three net sales milestones totaling $130 million are due on licensed products as are high single digit royalties due on aggregate, annual, worldwide net sales of licensed products.
Cincinnati Children’s Hospital Medical Center License Agreement
Pursuant to the terms of the Cincinnati license, Baergic paid an upfront fee of $0.2 million and as additional consideration for the license, Fortress transferred 624,922 common shares of Baergic, owned by Fortress, to Cincinnati as consideration for the Cincinnati License. In addition, pursuant to a separate subscription agreement, Baergic also provided Cincinnati with anti-dilution protection until the earliest to occur of (i) receipt of $15 million in aggregate gross proceeds from the sale of new securities to third-party investors, (ii) such time as Cincinnati holds fewer than 25% of shares issued in connection with the execution of the license agreement, (iii) closing of a change of control transaction, or (iv) immediately following the consummation of an IPO of Baergic. Shares issuable under the anti-dilution provisions are being accounted for as share-based payment transactions issued as part of the acquisition of the license.
Baergic valued the stock grant to Cincinnati utilizing a Required Rate of Return model to determine the weighted market value of invested capital, discounted by a lack of marketability of 44.6%, weighted average cost of capital of 20.5%, and net of debt utilized, and an option pricing model using a risk-free rate of 1.69%, a maturity of 5.0 years, and a volatility of 84% resulting in a value of $0.029 per share or $0.1 million on December 31, 2019.
Development milestone payments totaling approximately $6.5 million in the aggregate are due upon achievement of each milestone. Four commercial milestones totaling $21 million are due on licensed products as are low single digit royalties due on aggregate, annual, worldwide net sales of licensed products. Cincinnati is also entitled, upon approval of the first NDA for a licensed product in a Fragile X or Autism indication, to receive a number of shares of Baergic common stock equal to 3.5% of the fully-diluted capitalization of Baergic, calculated as of the date of the NDA approval.
For the years ended December 31, 2021 and 2020, Baergic recorded the expenses in research and development of approximately $3,000 and $11,000, respectively, in connection with the anti-dilution provisions in its licenses with AZ and Cincinnati.
F-11
Note 4 – Related Party Agreements
Founders Agreement and Management Services Agreement with Fortress
Effective March 9, 2017, the Company entered into a Founders Agreement with Fortress (the “Baergic Founders Agreement”). The Baergic Founders Agreement provides that, in exchange for the time and capital expended in the formation of Baergic and the identification of specific assets the acquisition of which result in the formation of a viable emerging growth life science company, Fortress will receive initial equity and certain rights described below. The Baergic Founders Agreement has a term of 15 years, which upon expiration automatically renews for successive one-year periods unless terminated by Fortress and the Company or a Change in Control (as defined in the Baergic Founders Agreement) occurs. Fortress was also issued, at founding, 250,000 shares of Class A Preferred Stock and 9,750,000 shares of Common Stock of Baergic.
As additional consideration under the Baergic Founders Agreement, Baergic will also: (i) pay an equity fee in shares of common stock, payable within five (5) business days of the closing of any equity or debt financing for Baergic that occurs after the effective date of the Baergic Founders Agreement and ending on the date when Fortress no longer has majority voting control in the Company’s voting equity, equal to two and one-half (2.5%) of the gross amount of any such equity or debt financing; and (ii) pay a cash fee equal to four and one-half percent (4.5%) of the Company’s annual net sales, payable on an annual basis, within ninety (90) days of the end of each calendar year. In the event of a Change in Control, the Company will pay a one-time change in control fee equal to five (5x) times the product of (A) net sales for the twelve (12) months immediately preceding the change in control and (B) four and one-half percent (4.5%).
Effective as of March 9, 2017, the Company entered into a Management Services Agreement (the “MSA”) with Fortress, pursuant to which Fortress renders management, advisory and consulting services to the Company. The MSA has an initial term of five years and is automatically renewed for successive five-year terms unless terminated in accordance with its provisions. Services provided under the MSA may include, without limitation, (i) advice and assistance concerning any and all aspects of the Company’s operations, clinical trials, financial planning and strategic transactions and financings and (ii) conducting relations on behalf of the Company with accountants, attorneys, financial advisors and other professionals (collectively, the “Services”). The Company is obligated to utilize clinical research services, medical education, communication and marketing services and investor relations/public relation services of companies or individuals designated by Fortress, provided those services are offered at market prices. However, the Company is not obligated to take or act upon any advice rendered from Fortress and Fortress shall not be liable for any of its actions or inactions based upon their advice. Pursuant to the MSA and the Company’s Certificate of Incorporation, Fortress and its affiliates, including all members of the Company’s Board of Directors, will have no fiduciary or other duty to communicate or present any corporate opportunities to the Company or to refrain from engaging in business that is similar to that of the Company. In consideration for the Services, the Company will pay Fortress an annual consulting fee of $0.5 million (the “Annual Consulting Fee”), payable in advance in equal quarterly installments on the first business day of each calendar quarter in each year, provided, however, that such Annual Consulting Fee shall be increased to $1.0 million for each calendar year in which the Company has net assets in excess of $100 million at the beginning of the calendar year. The Company records fifty percent of the Annual Consulting Fee in research and development expense and fifty percent in general and administrative expense in the Statement of Operations. The first Annual Consulting Fee payment shall be made on the first business day of the calendar quarter immediately following the completion of the first equity financing for the Company that is in excess of $10,000,000 in gross proceeds and shall include all amounts in arrears since inception through such payment as well as the amounts in advance for such quarterly payment. For the years ended December 31, 2021 and 2020, the Company recorded expense of $0.5 million and $0.5 million, respectively, related to this agreement.
As of December 31, 2021 and 2020, the Company’s total amounts payable pursuant to the Annual Consulting Fee were $1.0 million and $0.5 million, respectively, and are included in accounts payable and accrued expenses – related party.
Payables and Accrued Expenses Related Party
In the normal course of business Fortress pays for certain expenses on behalf of the Company. Such expenses are recognized in the statement of operations and added to the outstanding balance of the Fortress Note.
Certain parent costs associated with the activities of the Company have been allocated. The expense allocations to Baergic are employee compensation for R&D, finance and accounting service provided to the Company based on time spent on Baergic projects and activities. The allocations were based on assumptions that management believes are reasonable. For the years ended December 31, 2021 and 2020, the allocated expenses were approximately $96,000 and $91,000, respectively, and were recorded to general and administrative expenses and research and development expenses.
Notes Payable – Related Party (Fortress Note)
Fortress has funded the Company’s operations pursuant to the terms of a future advance promissory note (the “Fortress Note”) which matures on or before December 19, 2022. The Fortress Note is also immediately due and payable if (i) the Company commences any proceeding in bankruptcy or for dissolution, liquidation, winding-up, composition or other relief under bankruptcy laws; or (ii) such proceedings are commenced against the Company, or a receiver or trustee is appointed for the Company; or (iii) there is any material breach of any material covenant, warrant, representation, or other term or condition of the Fortress Note at any time that is not cured within the permitted time period. The Company is also permitted to prepay the note and accrued but unpaid interest at any time.
As of December 31, 2021, the Fortress Note totaled approximately $4.0 million. For the years ended December 31, 2021 and 2020, the Company recorded costs of approximately $0.3 million and $0.4 million, respectively of interest expense at 8% per annum, recorded in interest expense in the statement of operations.
As of December 31, 2021 and 2020, the Company’s accrued but unpaid interest under the Fortress Note were $0.6 million and $0.3 million, respectively, and are included in accrued interest – related party in the balance sheets.
Consulting Agreement with Dr. Jay Kranzler
The Company entered into a consulting agreement in December 2020 with Jay Kranzler, M.D., Ph.D. who is also a member of the Company’s Board of Directors. Dr. Kranzler is compensated $12,500 quarterly to perform consulting and advisory services to the Company in support of its strategic and corporate initiatives. The agreement may be terminated by either party upon three days written notice. The consulting fees are recognized in general and administrative expenses.
F-12
Note 5 – Accounts Payable and Accrued Expenses
At December 31, 2021 and 2020, accounts payable and accrued expenses consisted of the following:
| December 31, | December 31, | |||||||
| ($ in thousands) | 2021 | 2020 | ||||||
| Accounts payable and accrued expenses | $ | 3 | $ | 3 | ||||
| Accounts payable and accrued expenses – related party | 1,020 | 520 | ||||||
| Total accounts payable and accrued expenses | $ | 1,023 | $ | 523 | ||||
Note 6 – Stockholders’ Equity
The Company, in accordance with its certificate of incorporation, is authorized to issue (i) 50,000,000 common shares with a par value of $0.0001 per share and (ii) 2,000,000 shares of Preferred Stock, 250,000 of which are designated as Class A Preferred Stock and the remainder are undesignated Preferred Stock (see below Stock Issuances to Fortress and Note 4).
In connection with the Company’s formation, Fortress received 9,750,000 shares of the Common Stock and 250,000 shares of the Company’s Class A Preferred Stock, pursuant to the Founders Agreement. Fortress paid the par value of $1,000 in 2015. The fair value of the Company’s common shares approximated par value as no licenses had been transferred at that time. Dividends, if and when declared, are to be distributed pro-rata to the Class A Preferred and Common Stockholders.
Class A Preferred Stock
Class A Preferred Stock is identical to common stock other than as to voting rights, conversion rights and the PIK Dividend right (as described below). Each share of Class A Preferred Stock is entitled to vote the number of votes that is equal to one and one-tenth (1.1) times a fraction, the numerator of which is the sum of (A) the shares of outstanding Baergic common stock and (B) the whole shares of Baergic common stock into which the shares of outstanding Class A Preferred Stock are convertible and the denominator of which is the number of shares of outstanding Class A Preferred Stock. Thus, the Class A Preferred Stock will at all times constitute a voting majority. Each share of Class A Preferred Stock is convertible, at Fortress’ option, into one fully paid and nonassessable share of Baergic common stock, subject to certain adjustments. As holders of Class A Preferred Stock, Fortress will receive on each December 17 (each a “PIK Dividend Payment Date”) until the date all outstanding Class A Preferred Stock is converted into common stock, pro rata per share dividends paid in additional fully paid and nonassessable shares of common stock (“PIK Dividends”) such that the aggregate number of shares of common stock issued pursuant to such PIK Dividend is equal to two and one-half percent (2.5%) of Baergic’s fully-diluted outstanding capitalization on the date that is one (1) business day prior to any PIK Dividend Payment Date.
Common Stock
As of December 31, 2021, the Company’s authorized capital stock consists of 50,000,000 shares of common stock, with $0.0001 par value. The holders of Common Stock are entitled to one vote per share of Common Stock held.
F-13
In the event of our liquidation or dissolution, the holders of common stock are entitled to receive proportionately all assets available for distribution to stockholders after the payment of all debts and other liabilities and subject to the prior rights of any outstanding preferred stock. Holders of common stock have no preemptive, subscription, redemption or conversion rights. The rights, preferences and privileges of holders of common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock that we may designate and issue in the future.
Pursuant to the anti-dilution privileges to AZ and Cincinnati described in Note 3, AZ and Cincinnati were issued 316,411 and 78,989 shares of common stock in 2020, respectively, and 93,558 and 23,448 shares of common stock in 2021, respectively.
For the years ended December 31, 2021 and 2020, Baergic recorded the expenses in research and development of approximately $3,000 and $11,000, respectively, in connection with the anti-dilution provisions in its licenses with AZ and Cincinnati.
Pursuant to the terms of the Class A Preferred Stock and the PIK Dividends issuable on the PIK Dividend Payment date, Class A Preferred Stock holders were issued 340,620 shares of common stock in 2020 and 351,955 shares of common stock in 2021.
For the years ended December 31, 2021 and 2020, Baergic recorded the expenses in research and development of approximately $10,000 and $10,000, respectively, in connection with the PIK Dividends.
Restricted Stock Awards
The following table summarizes restricted stock award activities for the year ended December 31, 2021 and 2020:
| Weighted Average | ||||||||
| Grant Date Fair | ||||||||
| Number of Shares | Value | |||||||
| Nonvested at December 31, 2019 | — | $ | — | |||||
| Granted | 850,000 | $ | 0.03 | |||||
| Vested | (316,667 | ) | 0.03 | |||||
| Nonvested at December 31, 2020 | 533,333 | $ | 0.03 | |||||
| Granted | — | — | ||||||
| Vested | (116,667 | ) | 0.03 | |||||
| Nonvested at December 31, 2021 | 416,666 | $ | 0.03 | |||||
As of December 31, 2021, the Company had unrecognized stock-based compensation expense related to restricted stock of $12,000, which is expected to be recognized over a weighted average period of approximately 0.7 years.
The following table summarizes stock-based compensation expense for the years ended December 31, 2021 and 2020 (in thousands).
| For the year ended December 31, | |||||||
| 2021 | 2020 | ||||||
| General and administrative | $ | — | $ | — | |||
| Research and development | 4 | 12 | |||||
| Total stock-based compensation expense | $ | 4 | $ | 12 | |||
F-14
Stock Warrants
In 2018, Fortress closed a private placement of promissory notes (the “2018 Venture Notes”) through National Securities Corporation (“NSC”). Pursuant to the terms of the 2018 Venture Notes, Fortress advanced funds under the 2018 Ventures Notes to the Company for the acquisition and initial development costs for the AZ License and Cincinnati License. Such amounts are reflected in the Fortress Note balance on the balance sheet.
In connection with the advances under the 2018 Venture Notes, NSC received contingently issuable warrants to purchase the Company’s common stock equal to 25% of the total borrowing under the 2018 Venture Notes divided by the lowest price at which the Company sells its equity in its first third-party equity financing or in a change-of-control transaction. The warrants issued have a term of 10 years and an exercise price equal to the par value of the Company’s common stock. The fair value of the warrants are immaterial.
As of December 31, 2021, the total borrowing under the 2018 Venture Notes for the calculation of the contingently issuable warrants was $4.3 million. No additional advances can be made as Fortress extinguished the notes in August 2020.
Note 7 – Income Taxes
The Company records income taxes using the asset and liability method. Deferred income tax assets and liabilities are recognized for future tax effects attributable to temporary differences between the financial statement carrying amounts of existing assets and liabilities and their respective income tax bases, and operating loss and tax credit carryforwards. The Company establishes a valuation allowance if management believes it is more likely than not that the deferred tax assets will not be recovered based on an evaluation of objective verifiable evidence. Management has considered the Company’s history of book and tax losses incurred since inception, and the other positive and negative evidence, and has concluded that it is more likely than not that the Company will not realize the benefits of the net deferred tax assets as of December 31, 2021 and 2020.
For the years ended December 31, 2021 and 2020, income tax expense (or benefit) was $0 and $0, resulting in an effective tax rate of 0% and 0%. The effective tax rate remains the same due to a full valuation allowance in both years.
As of December 31, 2021, the Company had no unrecognized tax benefits and does not anticipate any significant change to the unrecognized tax benefit balance.
The Company has incurred net operating losses since inception. The Company has not reflected any benefit of such net operating loss carryforwards (“NOL”) in the accompanying financial statements and has established a valuation allowance of $1.2 million against its net deferred tax assets as of December 31, 2021. The valuation allowance increased by $0.2 million during the current year.
A reconciliation of the statutory U.S. federal rate to the Company’s effective tax rate is as follows:
| For the year ended December 31, | ||||||||
| 2021 | 2020 | |||||||
| Statutory federal income tax rate | 21 | % | 21 | % | ||||
| Credits | 0 | % | 0 | % | ||||
| Other | (0 | )% | (0 | )% | ||||
| Change in valuation allowance | (21 | )% | (21 | )% | ||||
| Income taxes provision (benefit) | — | — | ||||||
The components of the net deferred tax asset as of December 31, 2021 and 2020 are the following ($ in thousands):
| For the year ended December 31, | ||||||||
| 2021 | 2020 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss carryovers | $ | 309 | $ | 224 | ||||
| Amortization of license fees | 606 | 649 | ||||||
| Accruals and reserves | 215 | 109 | ||||||
| Tax credits | 1 | 0 | ||||||
| Business interest expense deduction limit | 64 | — | ||||||
| Total deferred tax assets | 1,195 | 982 | ||||||
| Less: valuation allowance | (1,193 | ) | (979 | ) | ||||
| Net deferred tax assets | $ | 2 | $ | 3 | ||||
| Deferred tax liabilities: | ||||||||
| Stock compensation | (2 | ) | (3 | ) | ||||
| Total deferred tax assets, net | $ | — | $ | — | ||||
The Company has incurred net operating losses (“NOLs”) since inception. At December 31, 2021, the Company had federal NOLs of $1.5 million, which can be carried forward indefinitely. The NOLs from tax years 2019 through 2020 remain open to examination (and adjustment) by the Internal Revenue Service and state taxing authorities. In addition, federal tax years ending December 31, 2019 and 2020 are open for assessment of federal taxes. The expiration of the statute of limitations related to the various state income and franchise tax returns varies by state.
F-15
Note 8 – Subsequent Events
Subsequent events have been evaluated through August 31, 2022, the date the financial statements were available to be issued.
On May 11, 2022, Avenue Therapeutics, Inc. (“Avenue”) entered into a stock contribution agreement (the “Contribution Agreement”) with Fortress pursuant to which Fortress agreed to transfer its ownership of a majority of the outstanding shares (common and preferred) in Baergic to Avenue. Under the Contribution Agreement, Fortress also agreed to assign to the Avenue certain intercompany agreements existing between Fortress and Baergic, including a Founders Agreement and Management Services Agreement. Consummation of the transactions contemplated by the Contribution Agreement is subject to the satisfaction of certain conditions precedent, including: (i) the closing of an equity financing by Avenue resulting in gross proceeds of no less than $7.5 million, (ii) the agreement by InvaGen to (A) have 100% of its shares in Avenue repurchased by Avenue and (B) terminate certain of the agreements into which it entered with Avenue and/or Fortress in connection with InvaGen’s 2019 equity investment in Avenue, which will eliminate certain negative consent rights of InvaGen over Avenue and restore certain rights and privileges of Fortress in Avenue, and (iii) the sustained listing of the Avenue’s Common Stock on Nasdaq. Avenue also entered into a Share Repurchase Agreement with InvaGen regarding the repurchase of the shares of Avenue’s Common Stock it holds and the termination of the Historic Rights, although no assurance can be given that the other required consents and approvals for the closing of the Contribution Agreement will be obtained or that the closing conditions will be satisfied in a timely manner or at all.
Note 9 – Events (Unaudited) Subsequent to the Date of the Report of Independent Auditor
Events subsequent to the date of the report of independent auditor have been evaluated through October 4, 2022, the date the financial statements were available to be reissued.
On October 2, 2022, Fortress agreed to forgive the notes payable and accrued interest between Baergic and Fortress. As of September 30, 2022, the notes payable – related party balance was approximately $4.3 million and the accrued interest – related party balance was approximately $0.8 million. Fortress will also forgive any additional notes payable and accrued interest through the date of the consummation of the transaction, which is not expected to be material.
F-16
Baergic Bio, Inc.
Financial Statements (unaudited)
June 30, 2022 and 2021
F-17
BAERGIC BIO, INC.
BALANCE SHEETS
(in thousands, except share amounts)
(unaudited)
| June 30, 2022 | December 31, 2021 | |||||||
| ASSETS | ||||||||
| Current Assets: | ||||||||
| Cash | $ | 11 | $ | 10 | ||||
| Total current assets | 11 | 10 | ||||||
| Total Assets | $ | 11 | $ | 10 | ||||
| LIABILITIES AND STOCKHOLDERS' DEFICIT | ||||||||
| Current Liabilities: | ||||||||
| Accounts payable and accrued expenses | 19 | 3 | ||||||
| Accounts payable and accrued expenses - related party | 1,270 | 1,020 | ||||||
| Accrued interest – related party | 722 | 564 | ||||||
| Notes payable – related party | 4,074 | 3,961 | ||||||
| Total current liabilities | 6,085 | 5,548 | ||||||
| Total Liabilities | 6,085 | 5,548 | ||||||
| Commitments and Contingencies | ||||||||
| Stockholders' Deficit | ||||||||
| Preferred Stock ($0.0001 par value), 2,000,000 shares authorized and 250,000 shares outstanding as of June 30, 2022 and December 31, 2021 | - | - | ||||||
| Common Stock ($0.0001 par value), 50,000,000 shares authorized and 14,297,173 shares issued and outstanding as of June 30, 2022 and December 31, 2021 | 1 | 1 | ||||||
| Additional paid-in capital | 141 | 140 | ||||||
| Accumulated deficit | (6,216 | ) | (5,679 | ) | ||||
| Total Stockholders’ Deficit | (6,074 | ) | (5,538 | ) | ||||
| Total Liabilities and Stockholders’ Deficit | $ | 11 | $ | 10 | ||||
See accompanying notes to financial statements.
F-18
BAERGIC BIO, INC.
STATEMENTS OF OPERATIONS
(in thousands)
(unaudited)
| For the six months ended June 30, | For the three months ended June 30, | |||||||||||||||
| 2022 | 2021 | 2022 | 2021 | |||||||||||||
| Operating expenses: | ||||||||||||||||
| Research and development | $ | 166 | $ | 165 | $ | 77 | $ | 82 | ||||||||
| General and administrative | 206 | 185 | 110 | 87 | ||||||||||||
| Total operating expenses | 372 | 350 | 187 | 169 | ||||||||||||
| Loss from operations | (372 | ) | (350 | ) | (187 | ) | (169 | ) | ||||||||
| Other expense: | ||||||||||||||||
| Interest expense - related party | 165 | 151 | 86 | 76 | ||||||||||||
| Total other expense | 165 | 151 | 86 | 76 | ||||||||||||
| Net Loss | $ | (537 | ) | $ | (501 | ) | $ | (273 | ) | $ | (245 | ) | ||||
See accompanying notes to financial statements.
F-19
BAERGIC BIO, INC.
STATEMENTS OF STOCKHOLDERS’ EQUITY
(in thousands, except share amounts)
(unaudited)
Three months ended June 30, 2022
| Preferred Shares | Common Shares | Additional Paid-in | Accumulated | Total Stockholders' | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | Deficit | ||||||||||||||||||||||
| Balances at March 31, 2022 | 250,000 | $ | - | 14,297,173 | $ | 1 | $ | 141 | $ | (5,943 | ) | $ | (5,799 | ) | ||||||||||||||
| Stock-based compensation expense | - | - | - | - | - | - | - | |||||||||||||||||||||
| Net loss | - | - | - | - | - | (273 | ) | (273 | ) | |||||||||||||||||||
| Balances at June 30, 2022 | 250,000 | $ | - | 14,297,173 | $ | 1 | $ | 141 | $ | (6,216 | ) | $ | (6,074 | ) | ||||||||||||||
Six months ended June 30, 2022
| Preferred Shares | Common Shares | Additional Paid-in | Accumulated | Total Stockholders' | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | Deficit | ||||||||||||||||||||||
| Balances at December 31, 2021 | 250,000 | $ | - | 14,297,173 | $ | 1 | $ | 140 | $ | (5,679 | ) | $ | (5,538 | ) | ||||||||||||||
| Stock-based compensation expense | - | - | - | - | 1 | - | 1 | |||||||||||||||||||||
| Net loss | - | - | - | - | - | (537 | ) | (537 | ) | |||||||||||||||||||
| Balances at June 30, 2022 | 250,000 | $ | - | 14,297,173 | $ | 1 | $ | 141 | $ | (6,216 | ) | $ | (6,074 | ) | ||||||||||||||
Three months ended June 30, 2021
| Preferred Shares | Common Shares | Additional Paid-in | Accumulated | Total Stockholders' | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | Deficit | ||||||||||||||||||||||
| Balances at March 31, 2021 | 250,000 | $ | - | 13,828,212 | $ | 1 | $ | 124 | $ | (4,922 | ) | $ | (4,797 | ) | ||||||||||||||
| Stock-based compensation expense | - | - | - | - | - | - | - | |||||||||||||||||||||
| Net loss | - | - | - | - | - | (246 | ) | (246 | ) | |||||||||||||||||||
| Balances at June 30, 2021 | 250,000 | $ | - | 13,828,212 | $ | 1 | $ | 124 | $ | (5,168 | ) | $ | (5,043 | ) | ||||||||||||||
Six months ended June 30, 2021
| Preferred Shares | Common Shares | Additional Paid-in | Accumulated | Total Stockholders' | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | Deficit | ||||||||||||||||||||||
| Balances at December 31, 2020 | 250,000 | $ | - | 13,828,212 | $ | 1 | $ | 122 | $ | (4,667 | ) | $ | (4,544 | ) | ||||||||||||||
| Stock-based compensation expense | - | - | - | - | 2 | - | - | |||||||||||||||||||||
| Net loss | - | - | - | - | - | (501 | ) | (501 | ) | |||||||||||||||||||
| Balances at June 30, 2021 | 250,000 | $ | - | 13,828,212 | $ | 1 | $ | 124 | $ | (5,168 | ) | $ | (5,045 | ) | ||||||||||||||
See accompanying notes to financial statements.
F-20
BAERGIC BIO, INC.
STATEMENTS OF CASH FLOWS
(in thousands)
(unaudited)
| For the six months
ended June 30, | ||||||||
| 2022 | 2021 | |||||||
| Cash Flows from Operating Activities: | ||||||||
| Net loss | $ | (537 | ) | $ | (501 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Interest expense | 165 | 151 | ||||||
| Stock-based compensation expense | 1 | 2 | ||||||
| Issuance of common shares - License Agreement | - | - | ||||||
| Issuance of common shares - Founders Agreement | - | - | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts payable and accrued expenses | 16 | - | ||||||
| Accounts payable and accrued expenses - related parties | 250 | 250 | ||||||
| Net cash used in operating activities | (105 | ) | (98 | ) | ||||
| Cash Flows from Financing Activities: | ||||||||
| Increase in notes payable – related party | 106 | 115 | ||||||
| Net cash provided by financing activities | 106 | 115 | ||||||
| Net increase in cash and cash equivalents | 1 | 17 | ||||||
| Cash and cash equivalents at beginning of period | 10 | 7 | ||||||
| Cash and cash equivalents at end of period | 11 | 24 | ||||||
See accompanying notes to financial statements.
F-21
Notes to Unaudited Interim Financial Statements
Note 1 - Organization and Description of Business
Baergic Bio, Inc. (the “Company” or “Baergic”) was incorporated in Delaware on June 10, 2015, however did not commence substantial operations until the execution of its license agreements in 2019. In December 2019, Baergic entered into two license agreements: (i) a License Agreement (the “AZ License”) with AstraZeneca AB (“AZ”) to acquire an exclusive license to patent and related intellectual property rights pertaining to their proprietary compound Gamma-aminobutyric acid receptor A alpha 2 & 3 (GABAA α2,3) positive allosteric modulators (collectively, the “AZ IP”); and (ii) an Exclusive License Agreement (the “Cincinnati License”) with Cincinnati Children’s Hospital Medical Center (“Cincinnati”) to acquire patent and related intellectual property rights pertaining to a GABA inhibitor program for neurological disorders (the “Cincinnati IP”). Baergic is a clinical-stage pharmaceutical company focused on the development of pharmaceutical products for the treatment of disorders associated with the central nervous system.
The Company is a majority-controlled subsidiary of Fortress Biotech, Inc. (“Fortress” or “Parent”).
Going Concern Considerations
Since inception, the Company has incurred operating losses and the Company’s operations have been financed primarily through an intercompany note from Fortress, on an as-needed basis. As of June 30, 2022, the Company’s stockholders’ deficit was $6.1 million. Further, the Company is not yet generating revenue and expects to continue to incur significant costs for the foreseeable future in pursuit of its development and financing plans and may never become profitable. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Note 2 - Significant Accounting Policies
Basis of Presentation
The Company’s financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”). The Company has no subsidiaries.
All intercompany transactions between Fortress and Baergic are classified as due from or due to related party in the financial statements.
In connection with the reissuance of the financial statements, the Company identified and corrected certain immaterial errors related to the overstatement of research and development, general and administrative, and interest expense – related party for the three months ended June 30, 2022 by approximately $14,000, $14,000, and $4,000, respectively, and for the three months ended June 30, 2021 by $13,000, $11,000, and $2,000, respectively.
The Company also corrected the December 31, 2021 accumulated deficit and total stockholders' deficit and net loss within the statement of stockholders' equity for the six months ended June 30, 2022 for an administrative error. The errors did not impact the balance sheet, statement of operations, or statement of cash flows.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Actual results could differ from those estimates.
Cash
The Company considers highly liquid investments with a maturity of three months or less when purchased to be cash equivalents. Cash at June 30, 2022 and 2021, consisted entirely of cash in institutions in the United States.
Research and Development Costs
Research and development costs are expensed as incurred. Advance payments for goods and services that will be used in future research and development activities are expensed when the activity has been performed or when the goods have been received rather than when the payment is made. Upfront and milestone payments due to third parties that perform research and development services on the Company’s behalf will be expensed as services are rendered or when the milestone is achieved.
Research and development costs primarily consist of personnel related expenses, including salaries, benefits, travel, and other related expenses, stock-based compensation, payments made to third parties for license and milestone costs related to in-licensed products and technology, payments made to third party contract research organizations for preclinical and clinical studies, investigative sites for clinical trials, consultants, the cost of acquiring and manufacturing clinical trial materials, costs associated with regulatory filings, laboratory costs and other supplies.
In accordance with Accounting Standards Codification (“ASC”) 730-10-25-1, Research and Development, costs incurred in obtaining licenses are charged to research and development expense if the rights licensed have not reached commercial feasibility and has no alternative future use. The licenses purchased by the Company require substantial completion of research and development, regulatory and marketing approval efforts to reach commercial feasibility and has no alternative future use. Accordingly, the total purchase price for the licenses acquired is reflected as research and development expenses in the Company’s Statements of Operations (see Note 3).
F-22
Annual PIK Dividend to Class A Preferred Stockholders
The Company issued 250,000 shares of Class A Preferred Stock to Fortress. The Class A Preferred Stock entitle the holder to an annual stock dividend equal to 2.5% of the fully diluted outstanding equity of the Company (“PIK Dividend”, see Note 6). The PIK Dividend was part of the consideration payable for formation of the Company and the identification of certain assets, including the licenses contributed to Baergic by Fortress (see Note 3).
Pursuant to the Certificate of Incorporation, the Company issued 351,955 shares of common stock to Fortress for the PIK Dividend, representing 2.5% of the fully-diluted outstanding equity of Baergic on December 16, 2021 and is recorded in the Statement of Stockholders’ Equity at June 30, 2022, as Issuance of common shares – Founders Agreement. The Company recorded no expense related to these issuable shares during the six months ending June 30, 2022.
Pursuant to the Certificate of Incorporation, the Company issued 340,620 shares of common stock to Fortress for the PIK Dividend, representing 2.5% of the fully-diluted outstanding equity of Baergic on December 16, 2020 and is recorded in the Statement of Stockholders’ Equity at June 30, 2021, as Issuance of common shares – Founders Agreement. The Company recorded no expense related to these issuable shares during the six months ending June 30, 2021.
Fair Value Measurement
The Company follows accounting guidance on fair value measurements for financial assets and liabilities measured at fair value on a recurring basis. Under the accounting guidance, fair value is defined as an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or a liability.
The accounting guidance requires fair value measurements be classified and disclosed in one of the following three categories:
| Level 1: | Quoted prices in active markets for identical assets or liabilities. |
| Level 2: | Observable inputs other than Level 1 prices, for similar assets or liabilities that are directly or indirectly observable in the marketplace. |
| Level 3: | Unobservable inputs which are supported by little or no market activity and that are financial instruments whose values are determined using pricing models, discounted cash flow methodologies, or similar techniques, as well as instruments for which the determination of fair value requires significant judgment or estimation. |
The fair value hierarchy also requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. Assets and liabilities measured at fair value are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. The Company’s assessment of the significance of a particular input to the fair value measurement in its entirety requires management to make judgments and consider factors specific to the asset or liability.
Stock-Based Compensation
The Company expenses stock-based compensation to employees and directors over the requisite service period based on the estimated grant-date fair value of the awards and forfeitures, which are recorded upon occurrence. Restricted stock awards and restricted stock unit awards are expensed under the straight-line method over the vesting period. Expense for awards with performance-based vesting criteria will be measured and recorded if and when it becomes probable that the performance criteria will be achieved.
F-23
Income Taxes
The Company records income taxes using the asset and liability method. Deferred income tax assets and liabilities are recognized for the future tax effects attributable to temporary differences between the financial statement carrying amounts of existing assets and liabilities and their respective income tax bases, and operating loss and tax credit carryforwards. The Company establishes a valuation allowance if management believes it is more likely than not that the deferred tax assets will not be recovered based on an evaluation of objective verifiable evidence. For tax positions that are more likely than not of being sustained upon audit, the Company recognizes the largest amount of the benefit that is greater than 50% likely of being realized. For tax positions that are not more likely than not of being sustained upon audit, the Company does not recognize any portion of the benefit.
Comprehensive Loss
The Company has no components of other comprehensive loss, and therefore, comprehensive loss equals net loss.
Recently Issued Accounting Pronouncements
In August 2020, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2020-06, Debt-Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging-Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity, which simplifies accounting for convertible instruments by removing major separation models required under current GAAP. The ASU removes certain settlement conditions that are required for equity contracts to qualify for the derivative scope exception and it also simplifies the diluted earnings per share calculation in certain areas. This guidance is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2023. Early adoption will be permitted. The Company is currently evaluating the impact of this standard on its financial statements.
Note 3 – License, Clinical Trial and Sponsored Research Agreements
License Agreements
AstraZeneca AB License Agreement
Pursuant to the terms of the AZ License, Baergic paid an upfront fee of $3.0 million and issued 2,492,192 common shares equal to 19.95% of Baergic to AZ as consideration for AZ License. In connection with the issuance of the shares, Baergic also provided AZ with anti-dilution protection until the earliest to occur of (i) receipt of $75 million in aggregate gross proceeds from the sale of new securities to third-party investors, (ii) such time as AZ holds fewer than 25% of shares issued in connection with the execution of the license agreement, (iii) closing of a change of control transaction, or (iv) immediately following the consummation of an IPO of Baergic. Shares issuable under the anti-dilution provisions are being accounted for as share-based payment transactions issued as part of the acquisition of the license.
Baergic valued the stock grant to AZ utilizing a Required Rate of Return model to determine the weighted market value of invested capital, discounted by a lack of marketability of 44.6%, weighted average cost of capital of 20.5%, and net of debt utilized, and an option pricing model using a risk-free rate of 1.69%, a maturity of 5.0 years, and a volatility of 84% resulting in a value of $0.029 per share or $0.1 million on December 31, 2019.
In addition, if Baergic issues shares of any class or series of Capital Stock that is senior to Common Stock, including any Class A Preferred Stock, then the shares of the Common Stock then held by AZ shall be convertible, at AZ’s sole option, into shares of such class or series of Capital Stock (“Exchange Right”). Such conversion shall require no additional consideration from AZ and will be into a number of shares of such class or series of Capital Stock to maintain AZ’s fully-diluted ownership percentage. The Exchange Right will terminate upon the earlier of (i) such time that AZ and its affiliates collectively hold fewer than 25% of the shares of Capital Stock issued to AZ in connection with the execution of the transaction, as adjusted for any stock combination, stock split, stock dividend, recapitalization or other similar transaction; (ii) the closing of a Complete Change of Control (a deemed liquidation event or an outside entity becoming the owner of 100% of the total voting power of the equity securities of Baergic then-outstanding that are entitle to vote); and (iii) immediately before the consummation of an IPO of Baergic.
Development milestone payments totaling approximately $75 million in the aggregate are due upon achievement of each milestone. Three net sales milestones totaling $130 million are due on licensed products as are high single digit royalties due on aggregate, annual, worldwide net sales of licensed products.
Cincinnati Children’s Hospital Medical Center License Agreement
Pursuant to the terms of the Cincinnati license, Baergic paid an upfront fee of $0.2 million and as additional consideration for the license, Fortress transferred 624,922 common shares of Baergic, owned by Fortress, to Cincinnati as consideration for the Cincinnati License. In addition, pursuant to a separate subscription agreement, Baergic also provided Cincinnati with anti-dilution protection until the earliest to occur of (i) receipt of $15 million in aggregate gross proceeds from the sale of new securities to third-party investors, (ii) such time as Cincinnati holds fewer than 25% of shares issued in connection with the execution of the license agreement, (iii) closing of a change of control transaction, or (iv) immediately following the consummation of an IPO of Baergic. Shares issuable under the anti-dilution provisions are being accounted for as share-based payment transactions issued as part of the acquisition of the license.
Baergic valued the stock grant to Cincinnati utilizing a Required Rate of Return model to determine the weighted market value of invested capital, discounted by a lack of marketability of 44.6%, weighted average cost of capital of 20.5%, and net of debt utilized, and an option pricing model using a risk-free rate of 1.69%, a maturity of 5.0 years, and a volatility of 84% resulting in a value of $0.029 per share or $0.1 million on December 31, 2019.
F-24
Development milestone payments totaling approximately $6.5 million in the aggregate are due upon achievement of each milestone. Four commercial milestones totaling $21 million are due on licensed products as are low single digit royalties due on aggregate, annual, worldwide net sales of licensed products. Cincinnati is also entitled, upon approval of the first NDA for a licensed product in a Fragile X or Autism indication, to receive a number of shares of Baergic common stock equal to 3.5% of the fully-diluted capitalization of Baergic, calculated as of the date of the NDA approval.
For the six months ended June 30, 2022 and 2021, Baergic recorded no expense, respectively, in connection with the anti-dilution provisions in its licenses with AZ and Cincinnati.
Note 4 – Related Party Agreements
Founders Agreement and Management Services Agreement with Fortress
Effective March 9, 2017, the Company entered into a Founders Agreement with Fortress (the “Baergic Founders Agreement”). The Baergic Founders Agreement provides that, in exchange for the time and capital expended in the formation of Baergic and the identification of specific assets the acquisition of which result in the formation of a viable emerging growth life science company, Fortress will receive initial equity and certain rights described below. The Baergic Founders Agreement has a term of 15 years, which upon expiration automatically renews for successive one-year periods unless terminated by Fortress and the Company or a Change in Control (as defined in the Baergic Founders Agreement) occurs. Fortress was also issued, at founding, 250,000 shares of Class A Preferred Stock and 9,750,000 shares of Common Stock of Baergic.
As additional consideration under the Baergic Founders Agreement, Baergic will also: (i) pay an equity fee in shares of common stock, payable within five (5) business days of the closing of any equity or debt financing for Baergic that occurs after the effective date of the Baergic Founders Agreement and ending on the date when Fortress no longer has majority voting control in the Company’s voting equity, equal to two and one-half (2.5%) of the gross amount of any such equity or debt financing; and (ii) pay a cash fee equal to four and one-half percent (4.5%) of the Company’s annual net sales, payable on an annual basis, within ninety (90) days of the end of each calendar year. In the event of a Change in Control, the Company will pay a one-time change in control fee equal to five (5x) times the product of (A) net sales for the twelve (12) months immediately preceding the change in control and (B) four and one-half percent (4.5%).
Effective as of March 9, 2017, the Company entered into a Management Services Agreement (the “MSA”) with Fortress, pursuant to which Fortress renders management, advisory and consulting services to the Company. The MSA has an initial term of five years and is automatically renewed for successive five-year terms unless terminated in accordance with its provisions. Services provided under the MSA may include, without limitation, (i) advice and assistance concerning any and all aspects of the Company’s operations, clinical trials, financial planning and strategic transactions and financings and (ii) conducting relations on behalf of the Company with accountants, attorneys, financial advisors and other professionals (collectively, the “Services”). The Company is obligated to utilize clinical research services, medical education, communication and marketing services and investor relations/public relation services of companies or individuals designated by Fortress, provided those services are offered at market prices. However, the Company is not obligated to take or act upon any advice rendered from Fortress and Fortress shall not be liable for any of its actions or inactions based upon their advice. Pursuant to the MSA and the Company’s Certificate of Incorporation, Fortress and its affiliates, including all members of the Company’s Board of Directors, will have no fiduciary or other duty to communicate or present any corporate opportunities to the Company or to refrain from engaging in business that is similar to that of the Company. In consideration for the Services, the Company will pay Fortress an annual consulting fee of $0.5 million (the “Annual Consulting Fee”), payable in advance in equal quarterly installments on the first business day of each calendar quarter in each year, provided, however, that such Annual Consulting Fee shall be increased to $1.0 million for each calendar year in which the Company has net assets in excess of $100 million at the beginning of the calendar year. The Company records fifty percent of the Annual Consulting Fee in research and development expense and fifty percent in general and administrative expense in the Statement of Operations. The first Annual Consulting Fee payment shall be made on the first business day of the calendar quarter immediately following the completion of the first equity financing for the Company that is in excess of $10,000,000 in gross proceeds and shall include all amounts in arrears since inception through such payment as well as the amounts in advance for such quarterly payment.
For the six months ended June 30, 2022 and 2021, the Company recorded expense of $0.3 million and $0.3 million, respectively, related to this agreement.
As of June 30, 2022 and 2021, the Company’s total amounts payable pursuant to the Annual Consulting Fee were $1.3 million and $0.7 million, respectively, and are included in accounts payable and accrued expenses – related party.
F-25
Payables and Accrued Expenses Related Party
In the normal course of business Fortress pays for certain expenses on behalf of the Company. Such expenses are recognized in the statement of operations and added to the outstanding balance of the Fortress Note.
Certain parent costs associated with the activities of the Company have been allocated. The expense allocations to Baergic are employee compensation for R&D, finance and accounting service provided to the Company based on time spent on Baergic projects and activities. The allocations were based on assumptions that management believes are reasonable. For the six months ended June 30, 2022, and 2021, the allocated expenses were approximately $56,000 and $48,000, respectively, and were recorded to general and administration expenses and research and development expenses.
Notes Payable – Related Party (Fortress Note)
Fortress has funded the Company’s operations pursuant to the terms of a future advance promissory note (the “Fortress Note”) which matures on or before December 19, 2022. The Fortress Note is also immediately due and payable if (i) the Company commences any proceeding in bankruptcy or for dissolution, liquidation, winding-up, composition or other relief under bankruptcy laws; or (ii) such proceedings are commenced against the Company, or a receiver or trustee is appointed for the Company; or (iii) there is any material breach of any material covenant, warrant, representation, or other term or condition of the Fortress Note at any time that is not cured within the permitted time period.
As of June 30, 2022, the Fortress Note totaled approximately $4.1 million. For the six months ended June 30, 2022, and 2021, the Company recorded costs of approximately $0.2 million and $0.2 million, respectively of interest expense at 8% per annum, recorded in interest expense in the statement of operations.
As of June 30, 2022, and 2021, the Company’s accrued but unpaid interest under the Fortress Note were $0.7 million and $0.4 million, respectively, and are included in accrued interest – related party on the balance sheets.
Consulting Agreement with Dr. Jay Kranzler
The Company entered into a consulting agreement in December 2020 with Jay Kranzler, M.D., Ph.D. who is also a member of the Company’s Board of Directors. Dr. Kranzler is compensated $12,500 quarterly to perform consulting and advisory services to the Company in support of its strategic and corporate initiatives. The agreement may be terminated by either party upon three days written notice. The consulting fees are recognized in general and administrative expenses.
Note 5 - Accounts Payable and Accrued Expenses
At June 30, 2022, and 2021, accounts payable and accrued expenses consisted of the following:
| As of June 30, | As of December 31, | |||||||
| ($ in thousands) | 2022 | 2021 | ||||||
| Accounts payable and accrued expenses | $ | 19 | $ | 3 | ||||
| Accounts payable and accrued expenses – related party | 1,270 | 1,020 | ||||||
| Total accounts payable and accrued expenses | $ | 1,289 | $ | 1,023 | ||||
Note 6 - Stockholders’ Equity
The Company, in accordance with its certificate of incorporation, is authorized to issue (i) 50,000,000 common shares with a par value of $0.0001 per share and (ii) 2,000,000 shares of Preferred Stock, 250,000 of which are designated as Class A Preferred Stock and the remainder are undesignated Preferred Stock (see below Stock Issuances to Fortress and Note 4).
In connection with the Company’s formation, Fortress received 9,750,000 shares of the Common Stock and 250,000 shares of the Company’s Class A Preferred Stock, pursuant to the Founders Agreement. Fortress paid the par value of $1,000 in 2015. The fair value of the Company’s common shares approximated par value as no licenses had been transferred at that time. Dividends, if and when declared, are to be distributed pro-rata to the Class A Preferred and Common Stockholders.
Class A Preferred Stock
Class A Preferred Stock is identical to common stock other than as to voting rights, conversion rights and the PIK Dividend right (as described below). Each share of Class A Preferred Stock is entitled to vote the number of votes that is equal to one and one-tenth (1.1) times a fraction, the numerator of which is the sum of (A) the shares of outstanding Baergic common stock and (B) the whole shares of Baergic common stock into which the shares of outstanding Class A Preferred Stock are convertible and the denominator of which is the number of shares of outstanding Class A Preferred Stock. Thus, the Class A Preferred Stock will at all times constitute a voting majority. Each share of Class A Preferred Stock is convertible, at Fortress’ option, into one fully paid and nonassessable share of Baergic common stock, subject to certain adjustments. As holders of Class A Preferred Stock, Fortress will receive on each December 17 (each a “PIK Dividend Payment Date”) until the date all outstanding Class A Preferred Stock is converted into common stock, pro rata per share dividends paid in additional fully paid and nonassessable shares of common stock (“PIK Dividends”) such that the aggregate number of shares of common stock issued pursuant to such PIK Dividend is equal to two and one-half percent (2.5%) of Baergic’s fully-diluted outstanding capitalization on the date that is one (1) business day prior to any PIK Dividend Payment Date.
F-26
Common Stock
As of June 30, 2022, the Company’s authorized capital stock consists of 50,000,000 shares of common stock, with $0.0001 par value. The holders of Common Stock are entitled to one vote per share of Common Stock held.
In the event of our liquidation or dissolution, the holders of common stock are entitled to receive proportionately all assets available for distribution to stockholders after the payment of all debts and other liabilities and subject to the prior rights of any outstanding preferred stock. Holders of common stock have no preemptive, subscription, redemption or conversion rights. The rights, preferences and privileges of holders of common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock that we may designate and issue in the future.
Pursuant to the anti-dilution privileges to AZ and Cincinnati described in Note 3, AZ and Cincinnati were issued no shares in the six months ended June 30, 2022, and 2021.
For the six months ended June 30, 2022, and 2021, Baergic recorded no expense in connection with the anti-dilution provisions in its licenses with AZ and Cincinnati.
Pursuant to the terms of the Class A Preferred Stock and the PIK Dividends issuable on the PIK Dividend Payment date, Class A Preferred Stock holders were issued no shares in the six months ended June 30, 2022, and 2021.
For the six months ended June 30, 2022, and 2021, Baergic recorded no expense in connection with the PIK Dividends.
Restricted Stock Awards
The following table summarizes restricted stock award activities for the six months ended June 30, 2022:
| Weighted Average | ||||||||
| Grant Date Fair | ||||||||
| Number of Shares | Value | |||||||
| Nonvested at December 31, 2021 | 416,666 | $ | 0.03 | |||||
| Granted | - | - | ||||||
| Vested | (16,666 | ) | 0.03 | |||||
| Nonvested at June 30, 2022 | 400,000 | $ | 0.03 | |||||
As of June 30, 2022, the Company had unrecognized stock-based compensation expense related to restricted stock of approximately $12,000, which is expected to be recognized over a weighted average period of approximately 0.6 years.
The following table summarizes stock-based compensation expense for the six months ended June 30, 2022, and 2021 (in thousands).
| For the six months ended June 30, | ||||||||
| 2022 | 2021 | |||||||
| General and administrative | $ | - | $ | - | ||||
| Research and development | 1 | 2 | ||||||
| Total stock-based compensation expense | $ | 1 | $ | 2 | ||||
Stock Warrants
In 2018, Fortress closed a private placement of promissory notes (the “2018 Venture Notes”) through National Securities Corporation (“NSC”). Pursuant to the terms of the 2018 Venture Notes, Fortress advanced funds under the 2018 Ventures Notes to the Company for the acquisition and initial development costs for the AZ License and Cincinnati License. Such amounts are reflected in the Fortress Note balance on the balance sheet.
In connection with the advances under the 2018 Venture Notes, NSC received contingently issuable warrants to purchase the Company’s common stock equal to 25% of the total borrowing under the 2018 Venture Notes divided by the lowest price at which the Company sells its equity in its first third-party equity financing or in a change-of-control transaction. The warrants issued have a term of 10 years and an exercise price equal to the par value of the Company’s common stock. The fair value of warrants are immaterial.
As of June 30, 2022, the total borrowing under the 2018 Venture Notes for the calculation of the contingently issuable warrants was $4.3 million. No additional advances can be made as Fortress extinguished the notes in August 2020.
F-27
Note 7 - Income Taxes
The Company records income taxes using the asset and liability method. Deferred income tax assets and liabilities are recognized for future tax effects attributable to temporary differences between the financial statement carrying amounts of existing assets and liabilities and their respective income tax bases, and operating loss and tax credit carryforwards. The Company establishes a valuation allowance if management believes it is more likely than not that the deferred tax assets will not be recovered based on an evaluation of objective verifiable evidence. Management has considered the Company’s history of book and tax income and losses incurred since inception, and the other positive and negative evidence, and has concluded that it is more likely than not that the Company will not realize the benefits of the net deferred tax assets as of June 30, 2022.
For the six months ended June 30, 2022, and 2021, income tax expense (or benefit) was $0 and $0, resulting in an effective tax rate of 0% and 0%. The effective tax rate remains the same due to a full valuation allowance in both years.
As of June 30, 2022, the Company had no unrecognized tax benefits and does not anticipate any significant change to the unrecognized tax benefit balance.
Note 8 – Subsequent Events
Subsequent events have been evaluated through August 31, 2022, the date the financial statements were available to be issued.
On May 11, 2022, Avenue Therapeutics, Inc. (“Avenue”) entered into a stock contribution agreement (the “Contribution Agreement”) with Fortress pursuant to which Fortress agreed to transfer its ownership of a majority of the outstanding shares (common and preferred) in Baergic to Avenue. Under the Contribution Agreement, Fortress also agreed to assign to the Avenue certain intercompany agreements existing between Fortress and Baergic, including a Founders Agreement and Management Services Agreement. Consummation of the transactions contemplated by the Contribution Agreement is subject to the satisfaction of certain conditions precedent, including: (i) the closing of an equity financing by Avenue resulting in gross proceeds of no less than $7.5 million, (ii) the agreement by InvaGen to (A) have 100% of its shares in Avenue repurchased by Avenue and (B) terminate certain of the agreements into which it entered with Avenue and/or Fortress in connection with InvaGen’s 2019 equity investment in Avenue, which will eliminate certain negative consent rights of InvaGen over Avenue and restore certain rights and privileges of Fortress in Avenue, and (iii) the sustained listing of the Avenue’s Common Stock on Nasdaq. Avenue also entered into a Share Repurchase Agreement with InvaGen regarding the repurchase of the shares of Avenue’s Common Stock it holds and the termination of the Historic Rights, although no assurance can be given that the other required consents and approvals for the closing of the Contribution Agreement will be obtained or that the closing conditions will be satisfied in a timely manner or at all.
Note 9 – Events Subsequent to the Date the Financial Statements Were Available to be Issued
Events subsequent to the date the financial statements were available to be issued have been evaluated through October 4, 2022, the date the financial statements were available to be reissued.
On October 2, 2022, Fortress agreed to forgive the notes payable and accrued interest between Baergic and Fortress. As of September 30, 2022, the notes payable – related party balance was approximately $4.3 million and the accrued interest – related party balance was approximately $0.8 million. Fortress will also forgive any additional notes payable and accrued interest through the date of the consummation of the transaction, which is not expected to be material.
F-28

2,652,065 Units, each consisting of one Share of Common Stock and one Warrant to purchase Shares of Common Stock
and
984,300 Pre-Funded Units, each consisting of one Pre-funded Warrant to purchase Shares of Common Stock and Warrant to purchase Shares of Common Stock
PROSPECTUS
October 6, 2022