
Filed Pursuant to Rule 433
Issuer Free Writing Prospectus dated October 5, 2023
Relating to Preliminary Prospectus dated September 29, 2023
Registration No. 333-274562

AVENUE THERAPEUTICS, INC. ⎸ NASDAQ: ATXI ⎸ OCTOBER 2023 Avenue Therapeutics Corporate Presentation

2 Disclaimers Cautionary Statement Regarding Forward - Looking Statements This presentation of Avenue Therapeutics, Inc . (“we,” “us,” “our,” “Avenue,” or the “Company”) contains predictive or “forward - looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 . All statements other than statements of current or historical fact contained in this presentation, including statements that express our intentions, plans, objectives, beliefs, expectations, strategies, predictions or any other statements relating to our future activities or other future events or conditions are forward - looking statements . The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “will,” “should,” “would” and similar expressions are intended to identify forward - looking statements . These statements are based on current expectations, estimates and projections made by management about our business, our industry and other conditions affecting our financial condition, results of operations or business prospects . These statements are not guarantees of future performance and involve risks, uncertainties and assumptions that are difficult to predict . Therefore, actual outcomes and results may differ materially from what is expressed or forecasted in, or implied by, the forward - looking statements due to numerous risks and uncertainties . Factors that could cause such outcomes and results to differ include, but are not limited to, risks and uncertainties arising from : the fact that we currently have no drug products for sale and that our success is dependent on our product candidates receiving regulatory approval and being successfully commercialized; the possibility that serious adverse or unacceptable side effects are identified during the development of our current or future product candidates, such that we would need to abandon or limit development of some of our product candidates; our ability to successfully integrate Baergic Bio, Inc . or develop BAER - 101 or AJ 201 ; the substantial doubt raised about our ability to continue as a going concern, which may hinder our ability to obtain future financing; the significant losses we have incurred since inception and our expectation that we will continue to incur losses for the foreseeable future; our need for substantial additional funding, which may not be available to us on acceptable terms, or at all, which unavailability could force us to delay, reduce or eliminate our product development programs or commercialization efforts; our reliance on third parties for several aspects of our operations; our reliance on clinical data and results obtained by third parties that could ultimately prove to be inaccurate or unreliable; the possibility that we may not receive regulatory approval for any or all of our product candidates, or that such approval may be significantly delayed due to scientific or regulatory reasons; the fact that even if one or more of our product candidates receives regulatory approval, they will remain subject to substantial regulatory scrutiny; the effects of current and future laws and regulations relating to fraud and abuse, false claims, transparency, health information privacy and security and other healthcare laws and regulations; the effects of competition for our product candidates and the potential for new products to emerge that provide different or better therapeutic alternatives for our targeted indications; the possibility that the government or third - party payors fail to provide adequate coverage and payment rates for our product candidates or any future products; our ability to establish sales and marketing capabilities or to enter into agreements with third parties to market and sell our product candidates; our exposure to potential product liability claims; our ability to secure adequate protection of our intellectual property and our potential inability to maintain sufficient patent protection for our technology and products; our

3 Disclaimers Cautionary Statement Regarding Forward - Looking Statements (cont’d) ability to maintain compliance with the obligations under our intellectual property licenses and funding arrangements with third parties, without which licenses and arrangements we could lose rights that are important to our business; the fact that Fortress controls a voting majority of our Common Stock and has rights to receive significant share grants annually; our ability to comply with the applicable listing standards and maintain our current listing for our Common Stock on The Nasdaq Capital Market ; and those risks discussed in our filings which we make with the Securities and Exchange Commission (the “SEC”) . Any forward - looking statements speak only as of the date on which they are made, and we undertake no obligation to publicly update or revise any forward - looking statements to reflect events or circumstances that may arise after the date of this presentation, except as required by applicable law . Investors should evaluate any statements made by us in light of these important factors . The information contained herein is intended to be reviewed in its totality, and any stipulations, conditions or provisos that apply to a given piece of information in one part of this presentation should be read as applying mutatis mutandis to every other instance of such information appearing herein . Important Information The Company has filed a registration statement (including a prospectus) with the SEC for the offering to which this communication relates . Before you invest, you should read the prospectus in that registration statement and other documents the Issuer has filed with the SEC for more complete information about the Company and this offering . You may get these documents for free by visiting EDGAR on the SEC Web site at www . sec . gov . Alternatively, the Company, any placement agent or any dealer participating in the offering will arrange to send you the prospectus if you request it by emailing syndicate@maximgrp . com or calling ( 212 ) 895 - 3745 .
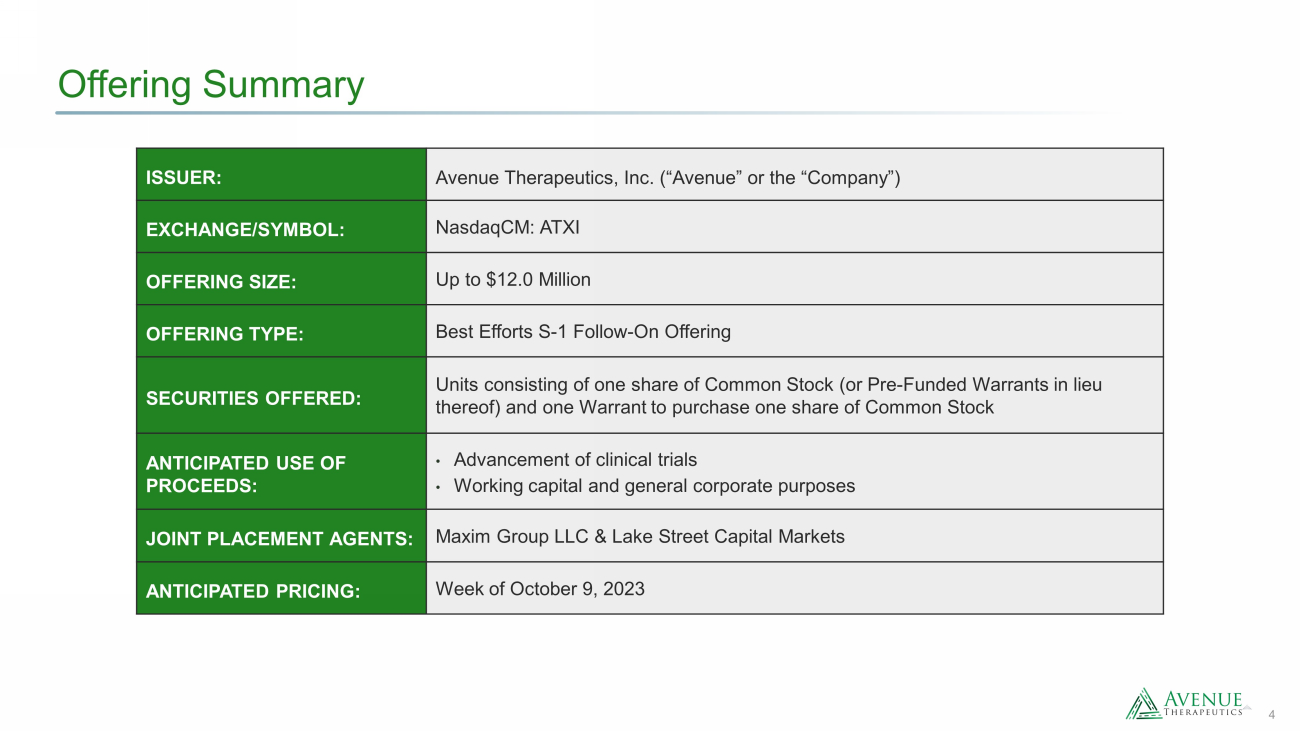
4 Offering Summary ISSUER: Avenue Therapeutics, Inc. (“Avenue” or the “Company”) EXCHANGE/SYMBOL: NasdaqCM : ATXI OFFERING SIZE: Up to $12.0 Million OFFERING TYPE : Best Efforts S - 1 Follow - On Offering SECURITIES OFFERED: Units consisting of one share of Common Stock (or Pre - Funded Warrants in lieu thereof) and one Warrant to purchase one share of Common Stock ANTICIPATED USE OF PROCEEDS: • Advancement of clinical trials • Working capital and general corporate purposes JOINT PLACEMENT AGENTS: Maxim Group LLC & Lake Street Capital Markets ANTICIPATED PRICING : Week of October 9, 2023

5 Executive Summary Two drugs are expected to deliver data in 2024 including the IV tramadol Phase 3 development program • Avenue Therapeutics is a company focused on developing therapies to treat neurologic diseases including acute hospital pain and a rare neuromuscular condition • IV tramadol has completed two Phase 3 efficacy trials with one remaining safety trial ‒ Phase 3 safety trial could be initiated in early 2024 and report data within 7 - 8 months to support a potential FDA approval in 2025 ‒ IV tramadol has been used globally ex - US for decades and addresses an unmet need in the U.S. postoperative pain market between stronger opioids and NSAIDs/Acetaminophen • AJ201 is currently in an ongoing Phase 1b/2a and has a proof - of - concept data readout in 2024 for treatment of a rare neurologic disease for which there is nothing approved outside of Japan ‒ Data readout in first half of 2024 for SBMA (spinal and bulbar muscular atrophy), also known as Kennedy’s Disease Note: Phase 3 tramadol trial initiation and data are subject to sufficient funding and/or partnering
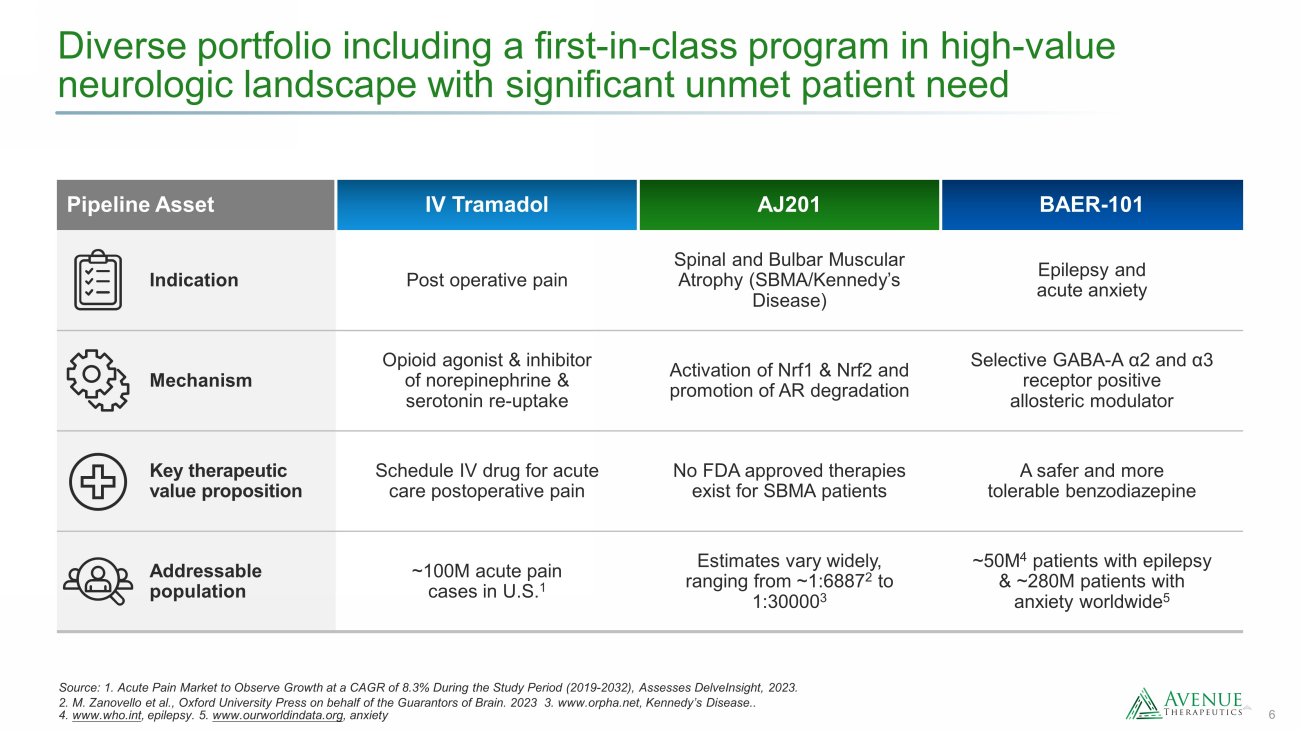
6 Diverse portfolio including a first - in - class program in high - value neurologic landscape with significant unmet patient need Source: 1. Acute Pain Market to Observe Growth at a CAGR of 8.3% During the Study Period (2019 - 2032), Assesses DelveInsight , 2023. 2. M. Zanovello et al., Oxford University Press on behalf of the Guarantors of Brain. 2023 3. www.orpha.net , Kennedy’s Disease.. 4. www.who.int , epilepsy. 5. www.ourworldindata.org , anxiety Pipeline Asset IV Tramadol AJ201 BAER - 101 Indication Post operative pain Spinal and Bulbar Muscular Atrophy (SBMA/Kennedy’s Disease) Epilepsy and acute anxiety Mechanism Opioid agonist & inhibitor of norepinephrine & serotonin re - uptake Activation of Nrf1 & Nrf2 and promotion of AR degradation Selective GABA - A α2 and α3 receptor positive allosteric modulator Key therapeutic value proposition Schedule IV drug for acute care postoperative pain No FDA approved therapies exist for SBMA patients A safer and more tolerable benzodiazepine Addressable population ~100M acute pain cases in U.S. 1 Estimates vary widely, ranging from ~ 1:6887 2 to 1:30000 3 ~50M 4 patients with epilepsy & ~280M patients with anxiety worldwide 5
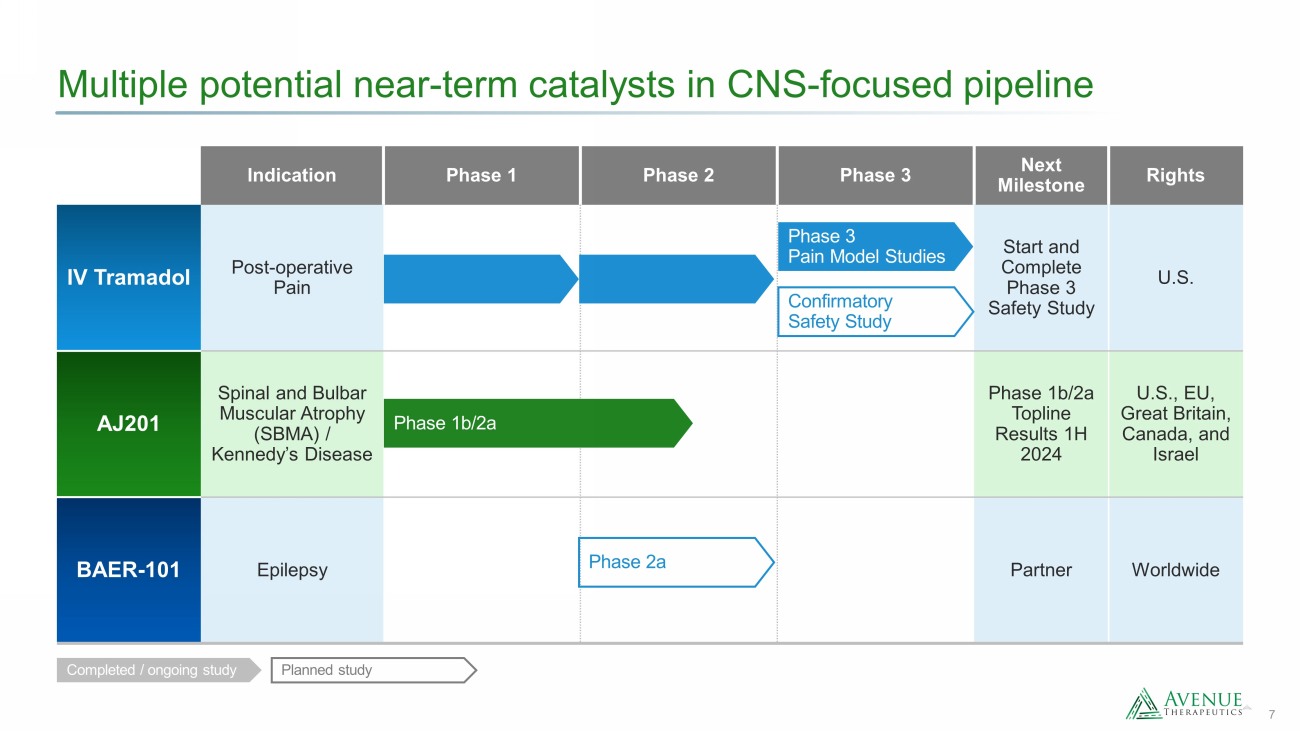
7 Multiple potential near - term catalysts in CNS - focused pipeline Indication Phase 1 Phase 2 Phase 3 Next Milestone Rights IV Tramadol Post - operative Pain Start and Complete Phase 3 Safety Study U.S. AJ201 Spinal and Bulbar Muscular Atrophy (SBMA) / Kennedy’s Disease Phase 1b/2a Topline Results 1H 2024 U.S., EU, Great Britain, Canada, and Israel BAER - 101 Epilepsy Partner Worldwide Completed / ongoing study Planned study Phase 3 Pain Model Studies Confirmatory Safety Study Phase 2a Phase 1b/2a
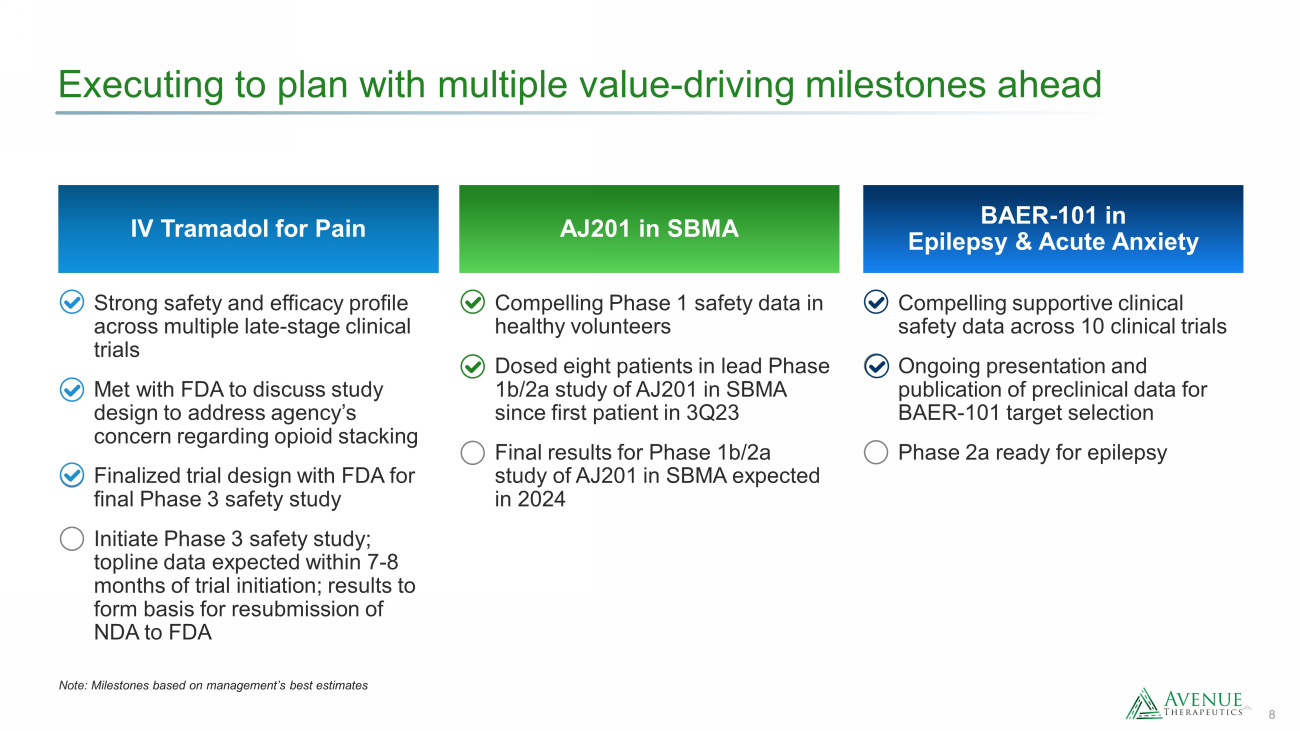
8 Executing to plan with multiple value - driving milestones ahead BAER - 101 in Epilepsy & Acute Anxiety AJ201 in SBMA Compelling Phase 1 safety data in healthy volunteers Dosed eight patients in lead Phase 1b/2a study of AJ201 in SBMA since first patient in 3Q23 Final results for Phase 1b/2a study of AJ201 in SBMA expected in 2024 Compelling supportive clinical safety data across 10 clinical trials Ongoing presentation and publication of preclinical data for BAER - 101 target selection Phase 2a ready for epilepsy IV Tramadol for Pain Strong safety and efficacy profile across multiple late - stage clinical trials Met with FDA to discuss study design to address agency’s concern regarding opioid stacking Finalized trial design with FDA for final Phase 3 safety study Initiate Phase 3 safety study; topline data expected within 7 - 8 months of trial initiation; results to form basis for resubmission of NDA to FDA Note: M ilestones based on management’s best estimates
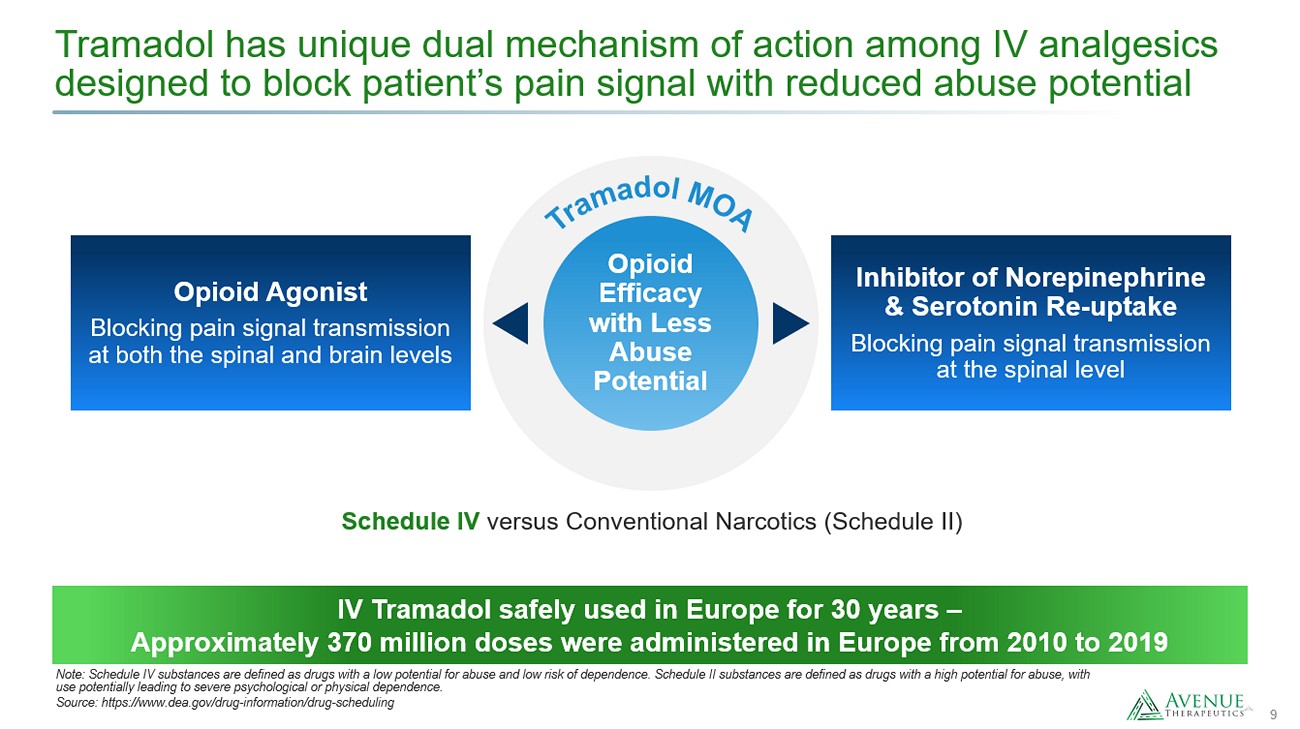
9 Tramadol has unique dual mechanism of action among IV analgesics designed to block patient’s pain signal with reduced abuse potential Note: Schedule IV substances are defined as drugs with a low potential for abuse and low risk of dependence. Schedule II subs tan ces are defined as drugs with a high potential for abuse, with use potentially leading to severe psychological or physical dependence. Source: https://www.dea.gov/drug - information/drug - scheduling IV Tramadol safely used in Europe for 30 years – Approximately 370 million doses were administered in Europe from 2010 to 2019 Opioid Efficacy with Less Abuse Potential Schedule IV versus Conventional Narcotics (Schedule II)
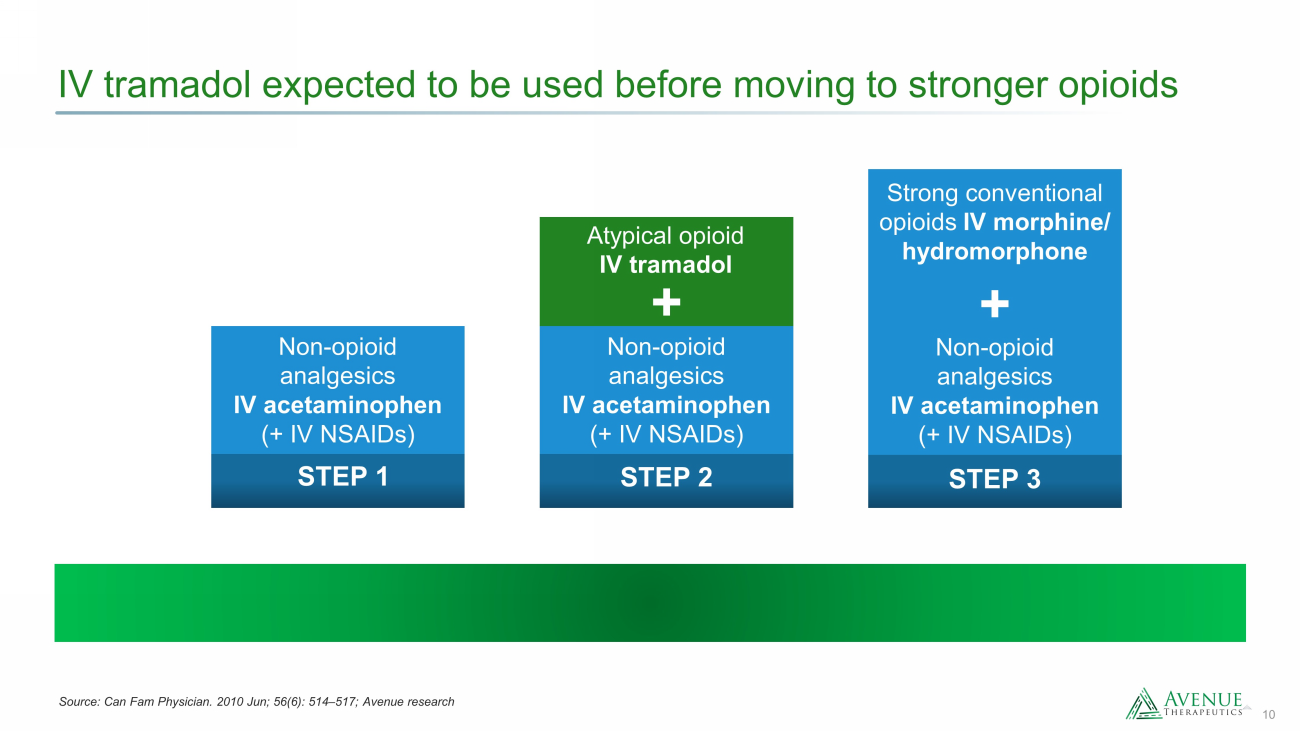
10 IV tramadol expected to be used before moving to stronger opioids STEP 1 Non - opioid analgesics IV acetaminophen (+ IV NSAIDs) STEP 2 Atypical opioid IV tramadol + Non - opioid analgesics IV acetaminophen (+ IV NSAIDs) + Strong conventional opioids IV morphine/ hydromorphone STEP 3 Non - opioid analgesics IV acetaminophen (+ IV NSAIDs) Source: Can Fam Physician. 2010 Jun; 56(6): 514 – 517; Avenue research
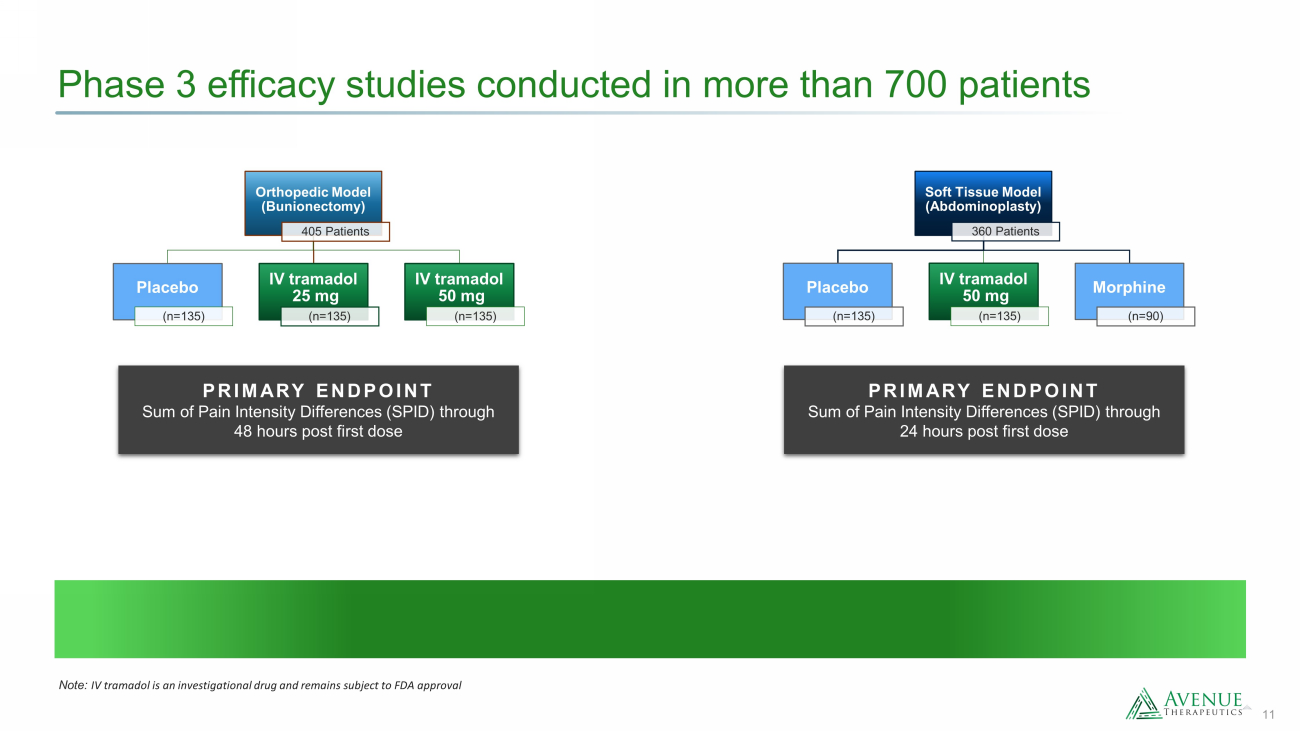
11 Phase 3 efficacy studies conducted in more than 700 patients Orthopedic Model (Bunionectomy) 405 Patients Placebo (n=135) IV tramadol 25 mg (n=135) IV tramadol 50 mg (n=135) Soft Tissue Model (Abdominoplasty) 360 Patients Placebo (n=135) IV tramadol 50 mg (n=135) Morphine (n=90) PRIMARY ENDPOINT Sum of Pain Intensity Differences (SPID) through 48 hours post first dose PRIMARY ENDPOINT Sum of Pain Intensity Differences (SPID) through 24 hours post first dose Note: IV tramadol is an investigational drug and remains subject to FDA approval
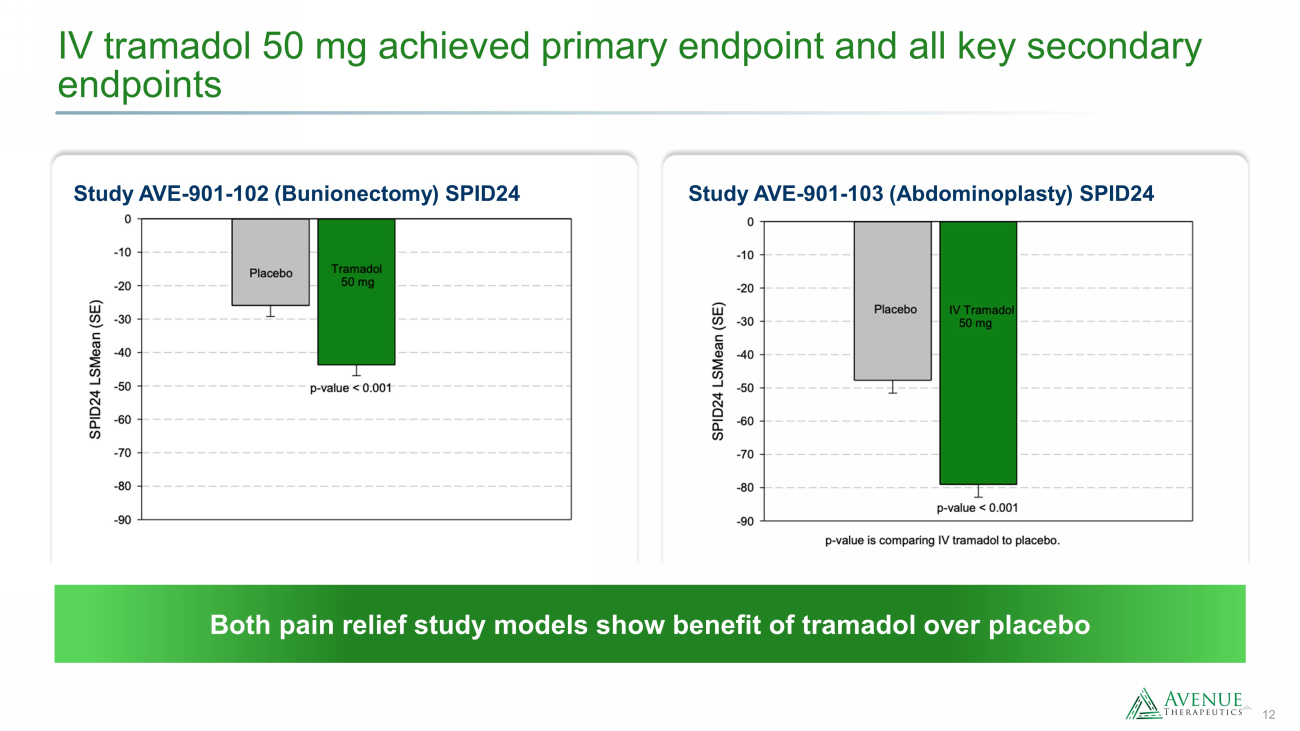
12 IV tramadol 50 mg achieved primary endpoint and all key secondary endpoints Study AVE - 901 - 103 (Abdominoplasty) SPID24 Study AVE - 901 - 102 (Bunionectomy) SPID24 Both pain relief study models show benefit of tramadol over placebo
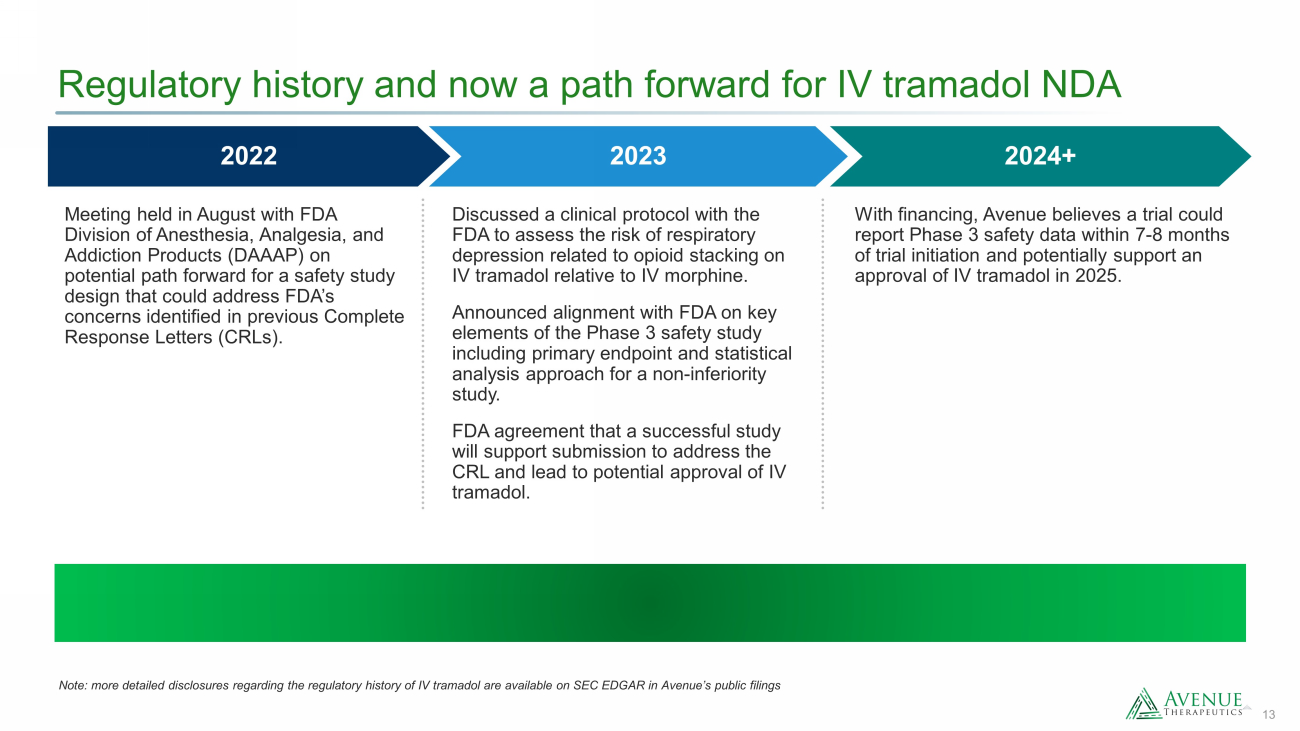
13 Regulatory history and now a path forward for IV tramadol NDA 2024+ 2022 2023 Meeting held in August with FDA Division of Anesthesia, Analgesia, and Addiction Products (DAAAP) on potential path forward for a safety study design that could address FDA’s concerns identified in previous Complete Response Letters (CRLs). Discussed a clinical protocol with the FDA to assess the risk of respiratory depression related to opioid stacking on IV tramadol relative to IV morphine. Announced alignment with FDA on key elements of the Phase 3 safety study including primary endpoint and statistical analysis approach for a non - inferiority study. FDA agreement that a successful study will support submission to address the CRL and lead to potential approval of IV tramadol. With financing, Avenue believes a trial could report Phase 3 safety data within 7 - 8 months of trial initiation and potentially support an approval of IV tramadol in 2025. Note: more detailed disclosures regarding the regulatory history of IV tramadol are available on SEC EDGAR in Avenue’s public fi lings
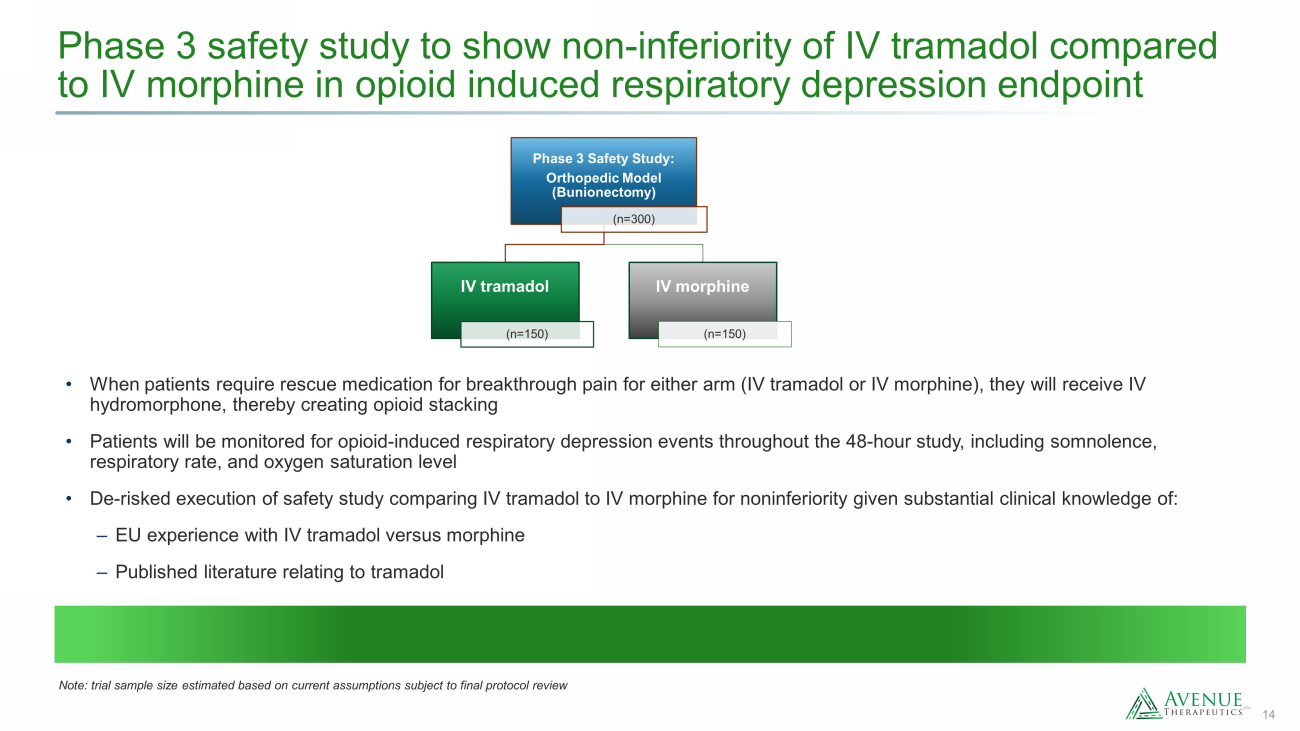
14 Phase 3 safety study to show non - inferiority of IV tramadol compared to IV morphine in opioid induced respiratory depression endpoint • When patients require rescue medication for breakthrough pain for either arm (IV tramadol or IV morphine), they will receive IV hydromorphone, thereby creating opioid stacking • Patients will be monitored for opioid - induced respiratory depression events throughout the 48 - hour study, including somnolence, respiratory rate, and oxygen saturation level • De - risked execution of safety study comparing IV tramadol to IV morphine for noninferiority given substantial clinical knowledge of: ‒ EU experience with IV tramadol versus morphine ‒ Published literature relating to tramadol Phase 3 Safety Study: Orthopedic Model (Bunionectomy) (n=300) IV tramadol (n=150) IV morphine (n=150) Note: trial sample size estimated based on current assumptions subject to final protocol review
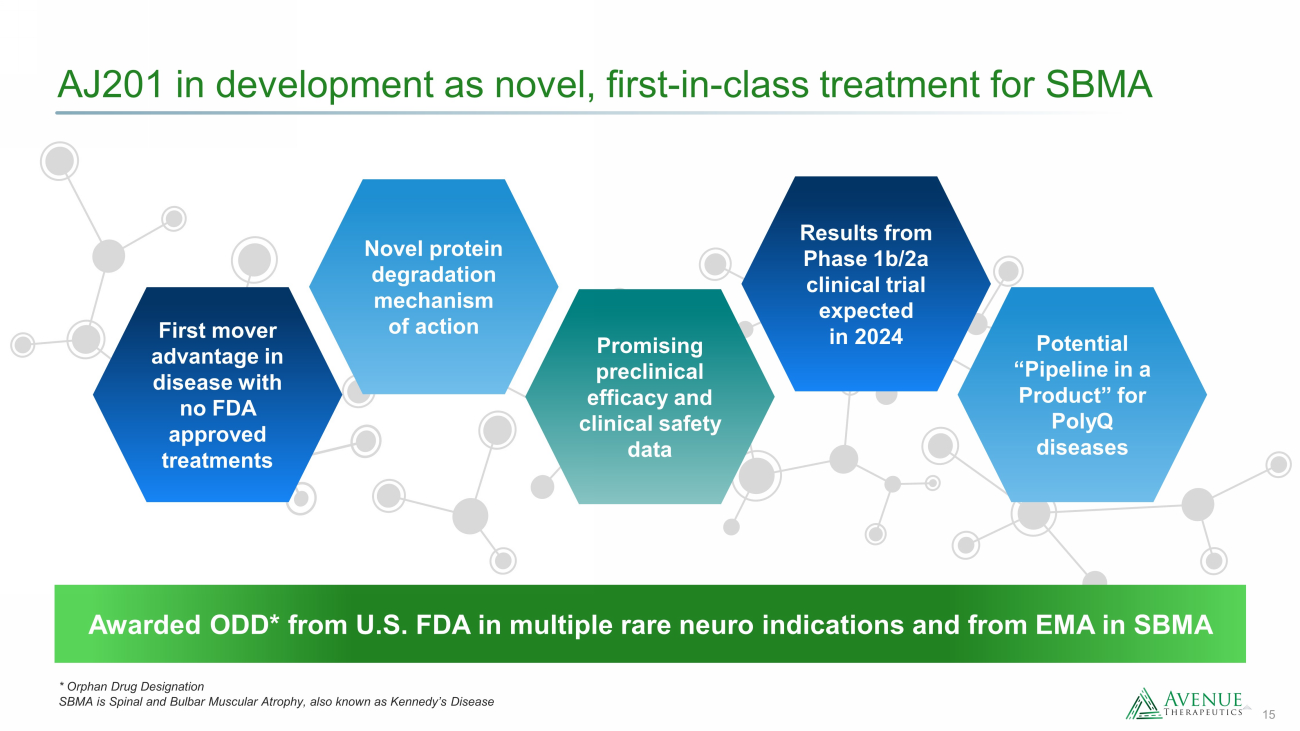
15 AJ201 in development as novel, first - in - class treatment for SBMA * Orphan Drug Designation SBMA is Spinal and Bulbar Muscular Atrophy, also known as Kennedy’s Disease Potential “Pipeline in a Product” for PolyQ diseases Promising preclinical efficacy and clinical safety data Novel protein degradation mechanism of action First mover advantage in disease with no FDA approved treatments Results from Phase 1b/2a clinical trial expected in 2024 Awarded ODD* from U.S. FDA in multiple rare neuro indications and from EMA in SBMA
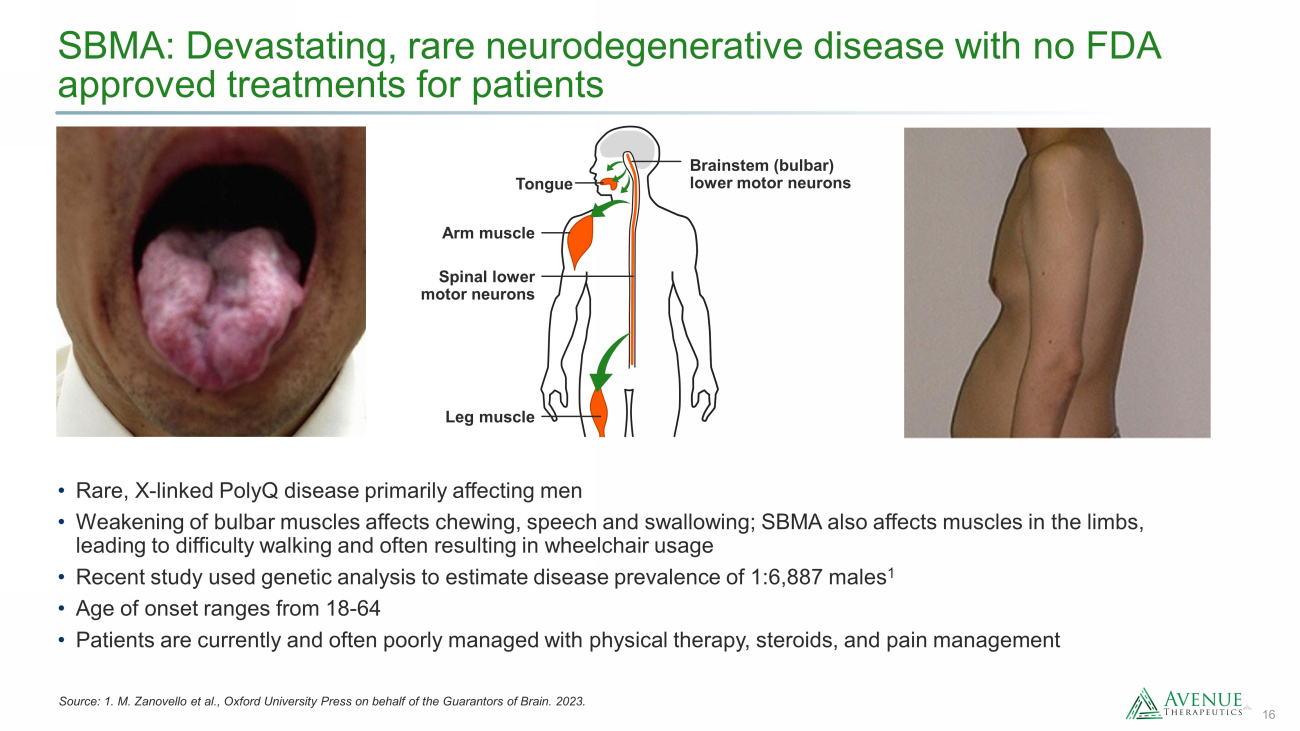
16 SBMA: Devastating, rare neurodegenerative disease with no FDA approved treatments for patients Source: 1. M. Zanovello et al., Oxford University Press on behalf of the Guarantors of Brain. 2023. • Rare, X - linked PolyQ disease primarily affecting men • Weakening of bulbar muscles affects chewing, speech and swallowing; SBMA also affects muscles in the limbs, leading to difficulty walking and often resulting in wheelchair usage • Recent study used genetic analysis to estimate disease prevalence of 1:6,887 males 1 • Age of onset ranges from 18 - 64 • Patients are currently and often poorly managed with physical therapy, steroids, and pain management Brainstem (bulbar) lower motor neurons Tongue Arm muscle Leg muscle Spinal lower motor neurons
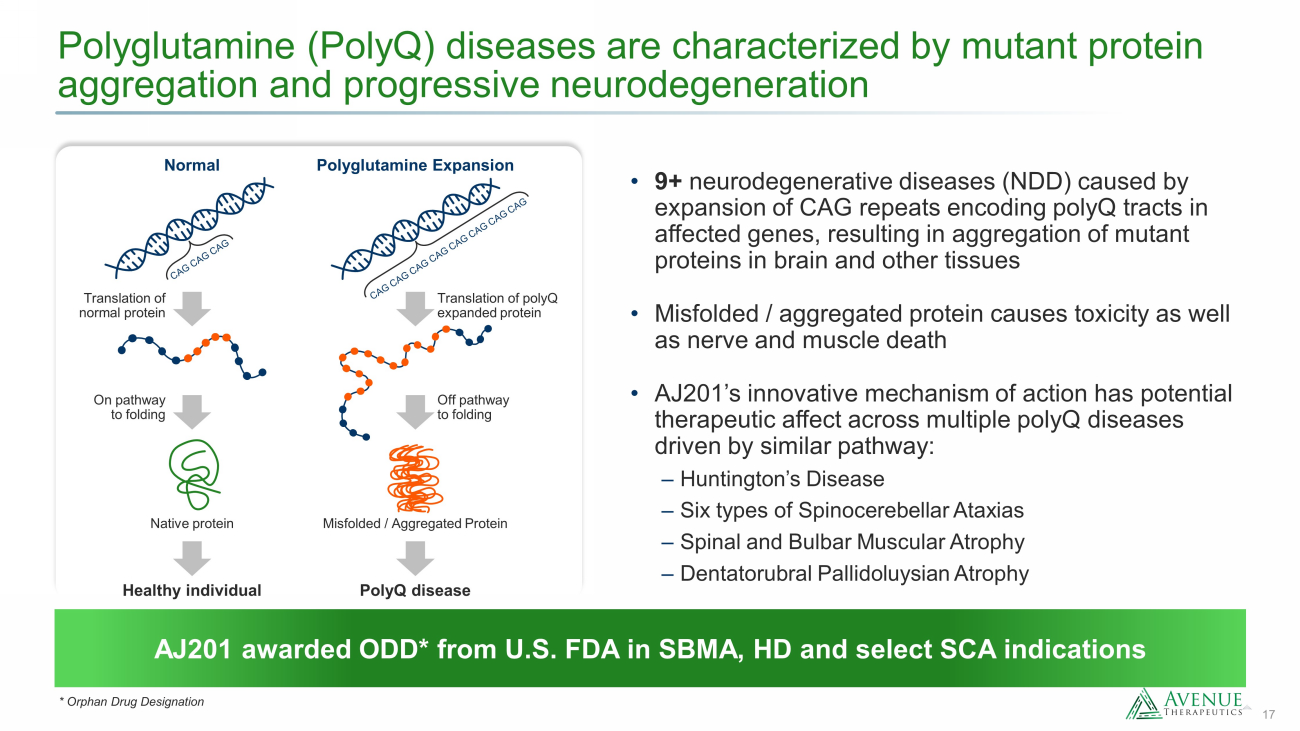
17 AJ201 awarded ODD* from U.S. FDA in SBMA, HD and select SCA indications Polyglutamine (PolyQ) diseases are characterized by mutant protein aggregation and progressive neurodegeneration * Orphan Drug Designation • 9+ neurodegenerative diseases (NDD) caused by expansion of CAG repeats encoding polyQ tracts in affected genes, resulting in aggregation of mutant proteins in brain and other tissues • Misfolded / aggregated protein causes toxicity as well as nerve and muscle death • AJ201’s innovative mechanism of action has potential therapeutic affect across multiple polyQ diseases driven by similar pathway: ‒ Huntington’s Disease ‒ Six types of Spinocerebellar Ataxias ‒ Spinal and Bulbar Muscular Atrophy ‒ Dentatorubral Pallidoluysian Atrophy Normal Polyglutamine Expansion Native protein Healthy individual PolyQ disease Off pathway to folding On pathway to folding Translation of normal protein Misfolded / Aggregated Protein Translation of polyQ expanded protein
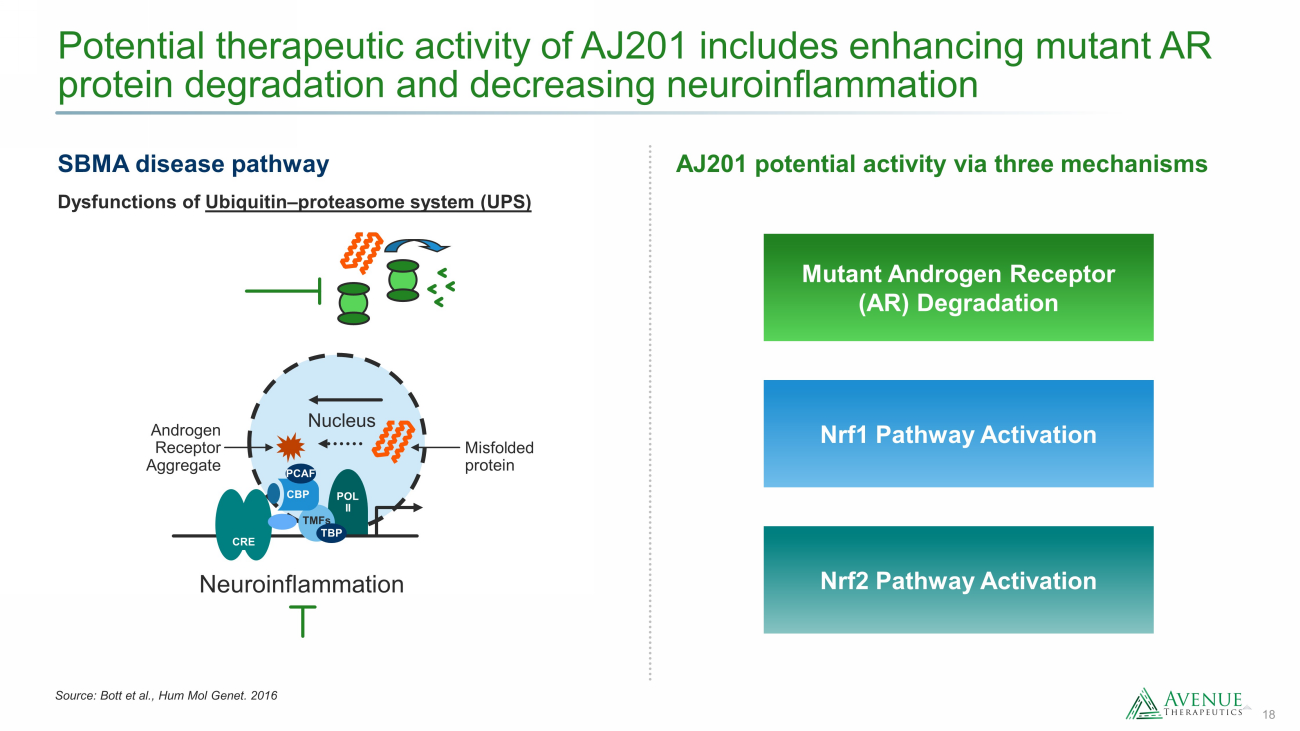
18 Potential therapeutic activity of AJ201 includes enhancing mutant AR protein degradation and decreasing neuroinflammation Source: Bott et al., Hum Mol Genet. 2016 SBMA disease pathway Nrf1 Pathway Activation Nrf2 Pathway Activation Mutant Androgen Receptor (AR) Degradation AJ201 potential activity via three mechanisms Dysfunctions of Ubiquitin – proteasome system (UPS) Androgen Receptor Aggregate Misfolded protein Nucleus Neuroinflammation POL II TMFs TBP CBP PCAF CRE
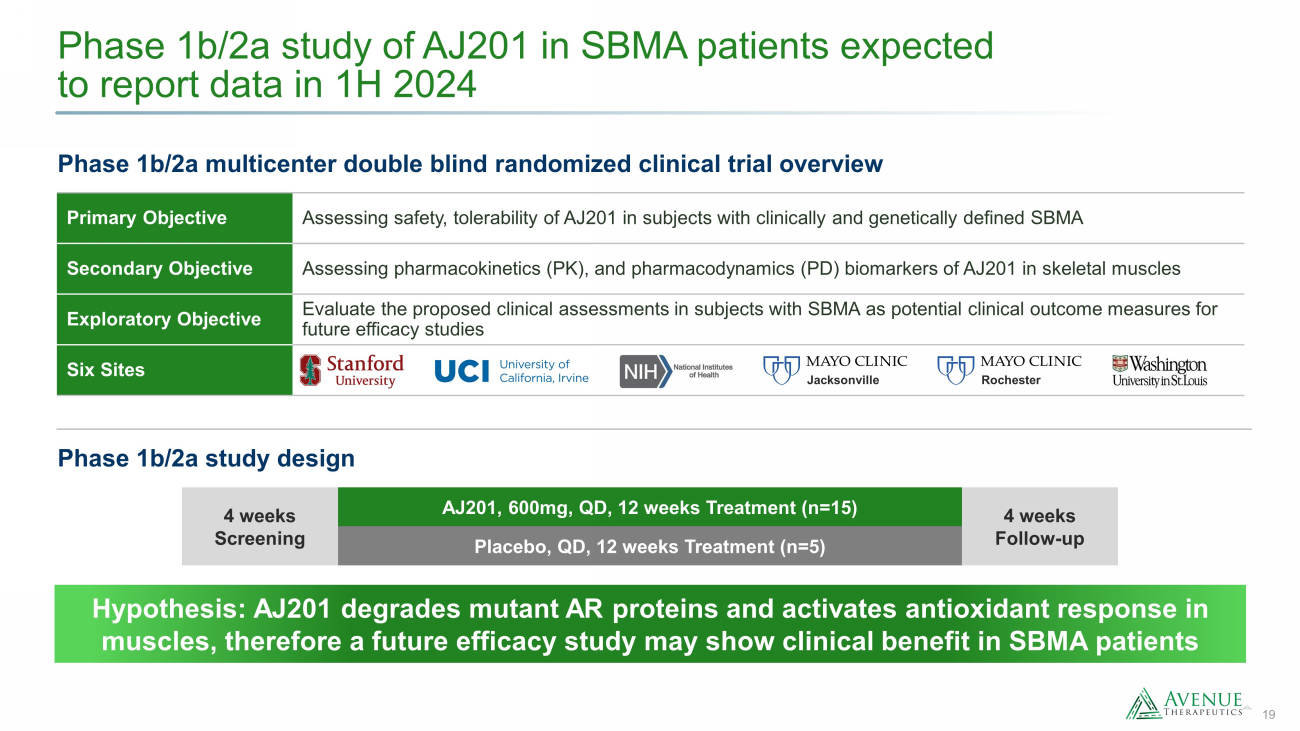
19 Phase 1b/2a study of AJ201 in SBMA patients expected to report data in 1H 2024 Phase 1b/2a multicenter double blind randomized clinical trial overview 4 weeks Screening AJ201, 600mg, QD, 12 weeks Treatment (n=15) 4 weeks Follow - up Placebo, QD, 12 weeks Treatment (n=5) Primary Objective Assessing safety, tolerability of AJ201 in subjects with clinically and genetically defined SBMA Secondary Objective Assessing pharmacokinetics (PK), and pharmacodynamics (PD) biomarkers of AJ201 in skeletal muscles Exploratory Objective Evaluate the proposed clinical assessments in subjects with SBMA as potential clinical outcome measures for future efficacy studies Six Sites Hypothesis: AJ201 degrades mutant AR proteins and activates antioxidant response in muscles, therefore a future efficacy study may show clinical benefit in SBMA patients Phase 1b/2a study design Jacksonville Rochester
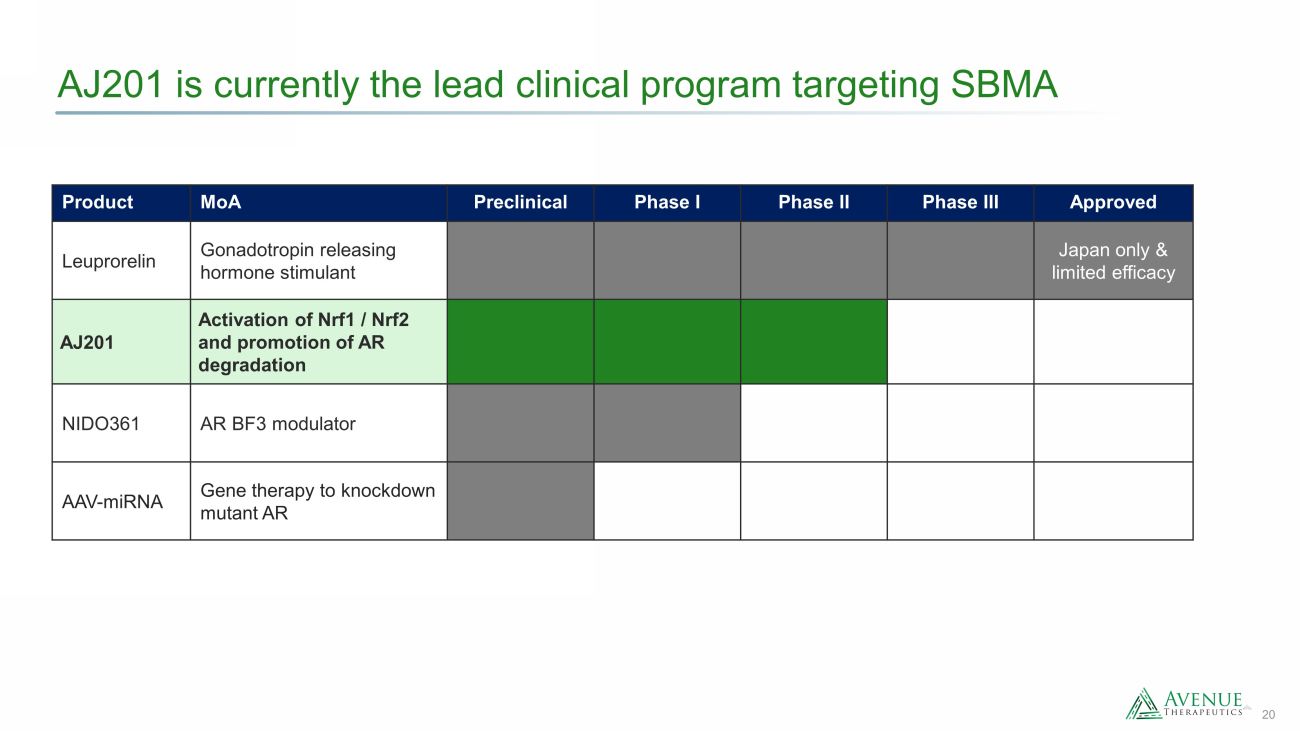
20 AJ201 is currently the lead clinical program targeting SBMA Product MoA Preclinical Phase I Phase II Phase III Approved Leuprorelin Gonadotropin releasing hormone stimulant Japan only & limited efficacy AJ201 Activation of Nrf1 / Nrf2 and promotion of AR degradation NIDO361 AR BF3 modulator AAV - miRNA Gene therapy to knockdown mutant AR
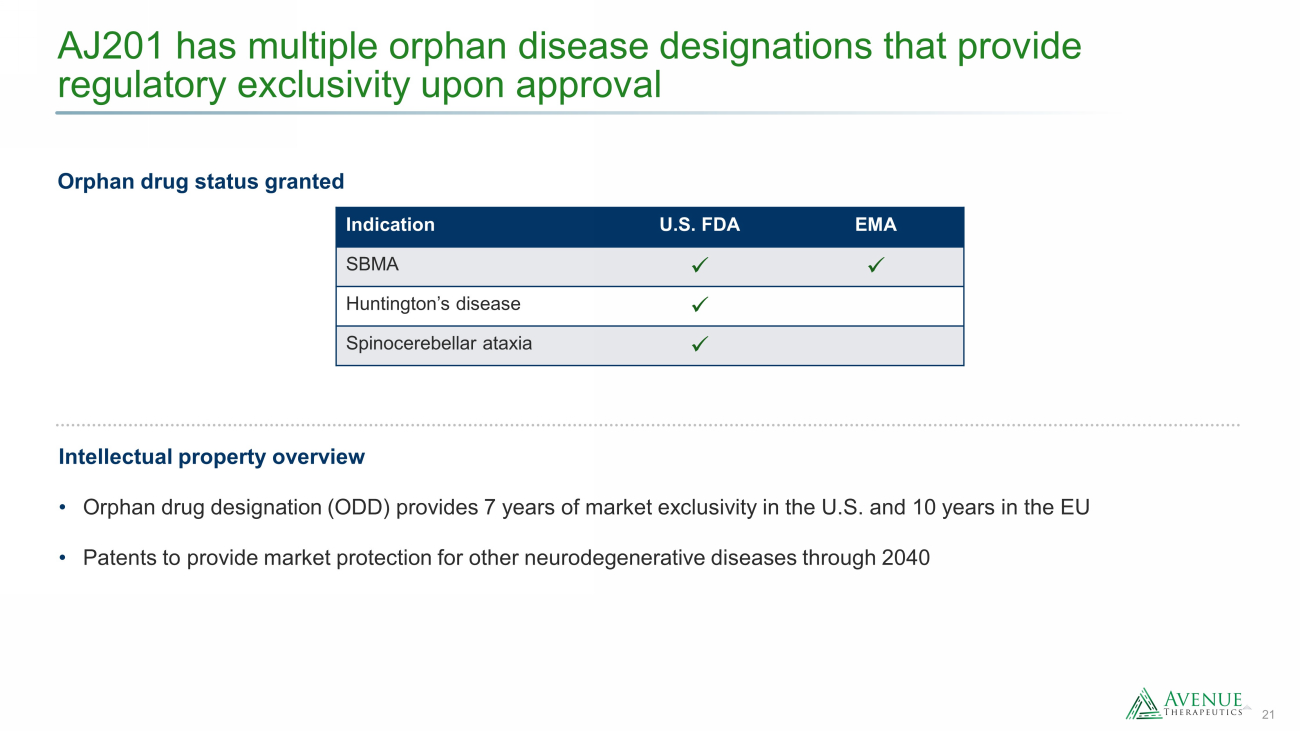
21 AJ201 has multiple orphan disease designations that provide regulatory exclusivity upon approval Orphan drug status granted Intellectual property overview • Orphan drug designation (ODD) provides 7 years of market exclusivity in the U.S. and 10 years in the EU • Patents to provide market protection for other neurodegenerative diseases through 2040 Indication U.S. FDA EMA SBMA x x Huntington’s disease x Spinocerebellar ataxia x
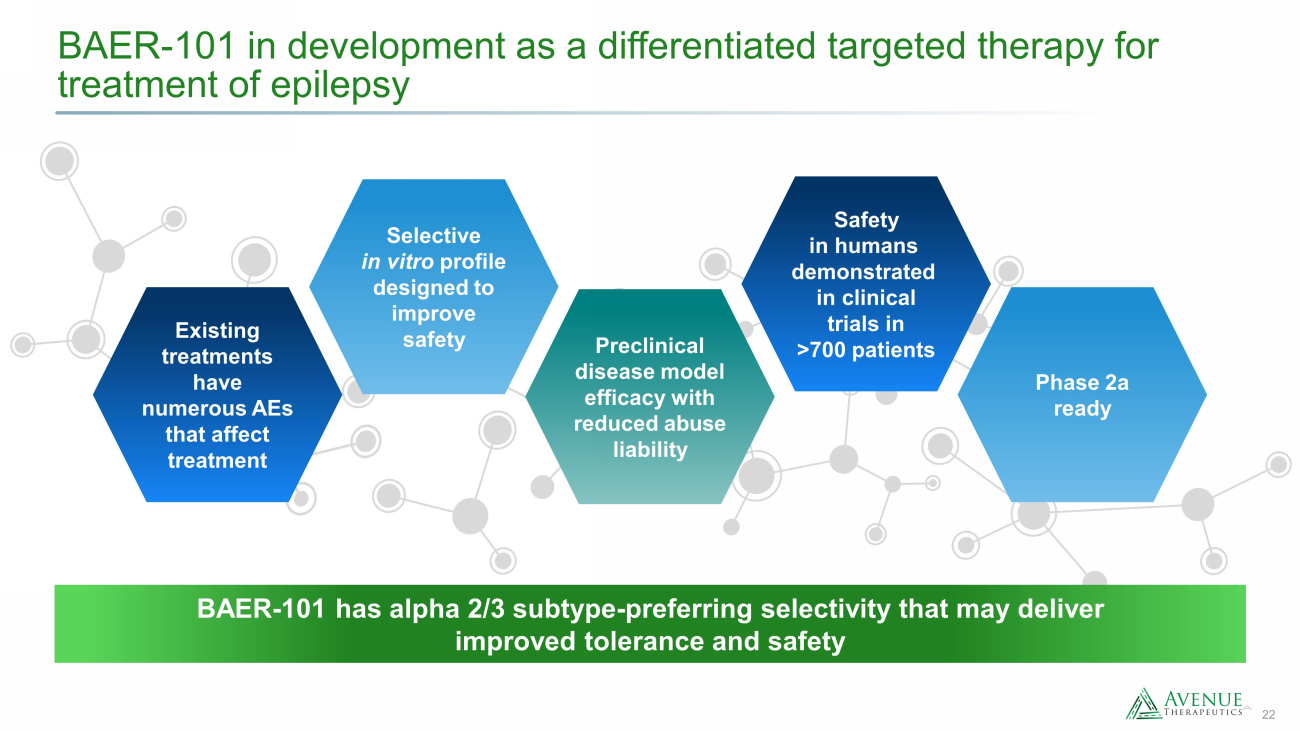
22 BAER - 101 in development as a differentiated targeted therapy for treatment of epilepsy Phase 2a ready Preclinical disease model efficacy with reduced abuse liability Selective in vitro profile designed to improve safety Existing treatments have numerous AEs that affect treatment Safety in humans demonstrated in clinical trials in >700 patients BAER - 101 has alpha 2/3 subtype - preferring selectivity that may deliver improved tolerance and safety
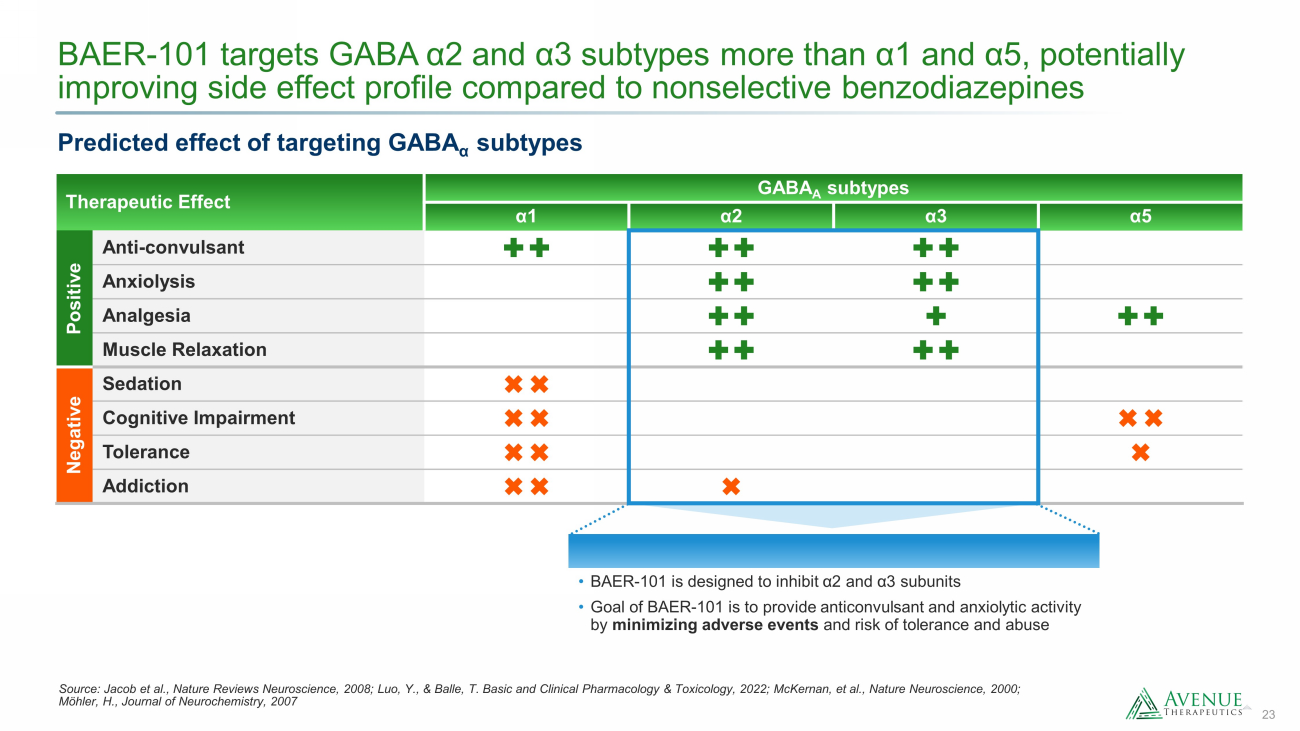
23 BAER - 101 targets GABA α2 and α3 subtypes more than α1 and α5, potentially improving side effect profile compared to nonselective benzodiazepines Source: Jacob et al., Nature Reviews Neuroscience, 2008; Luo, Y., & Balle, T. Basic and Clinical Pharmacology & Toxicology, 2 022 ; McKernan, et al., Nature Neuroscience, 2000; Möhler, H., Journal of Neurochemistry, 2007 Therapeutic Effect GABA A subtypes α 1 α 2 α 3 α 5 Positive Anti - convulsant Anxiolysis Analgesia Muscle Relaxation Negative Sedation Cognitive Impairment Tolerance Addiction Predicted effect of targeting GABA α subtypes • BAER - 101 is designed to inhibit α 2 and α 3 subunits • Goal of BAER - 101 is to provide anticonvulsant and anxiolytic activity by minimizing adverse events and risk of tolerance and abuse
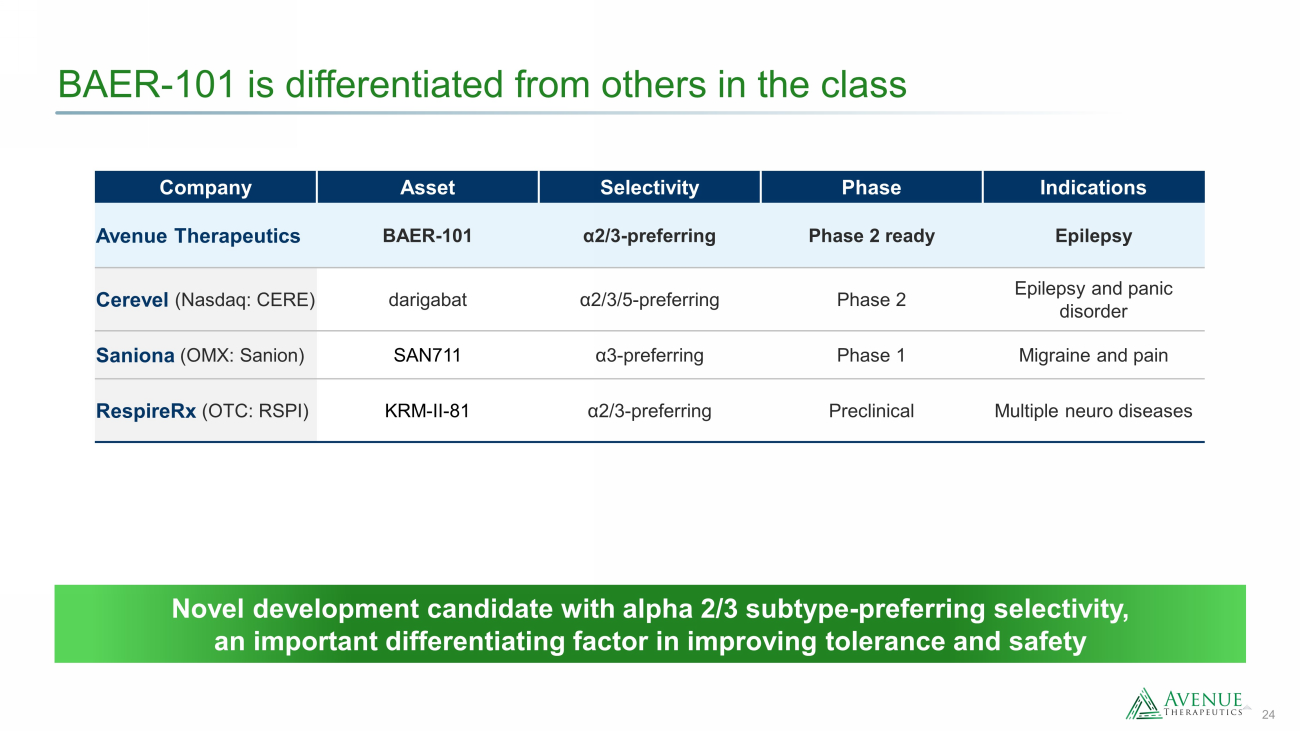
24 BAER - 101 is differentiated from others in the class Novel development candidate with alpha 2/3 subtype - preferring selectivity, an important differentiating factor in improving tolerance and safety Company Asset Selectivity Phase Indications Avenue Therapeutics BAER - 101 α2/3 - preferring Phase 2 ready Epilepsy Cerevel (Nasdaq: CERE) darigabat α2/3/5 - preferring Phase 2 Epilepsy and panic disorder Saniona (OMX: Sanion ) SAN711 α3 - preferring Phase 1 Migraine and pain RespireRx (OTC: RSPI) KRM - II - 81 α2/3 - preferring Preclinical Multiple neuro diseases
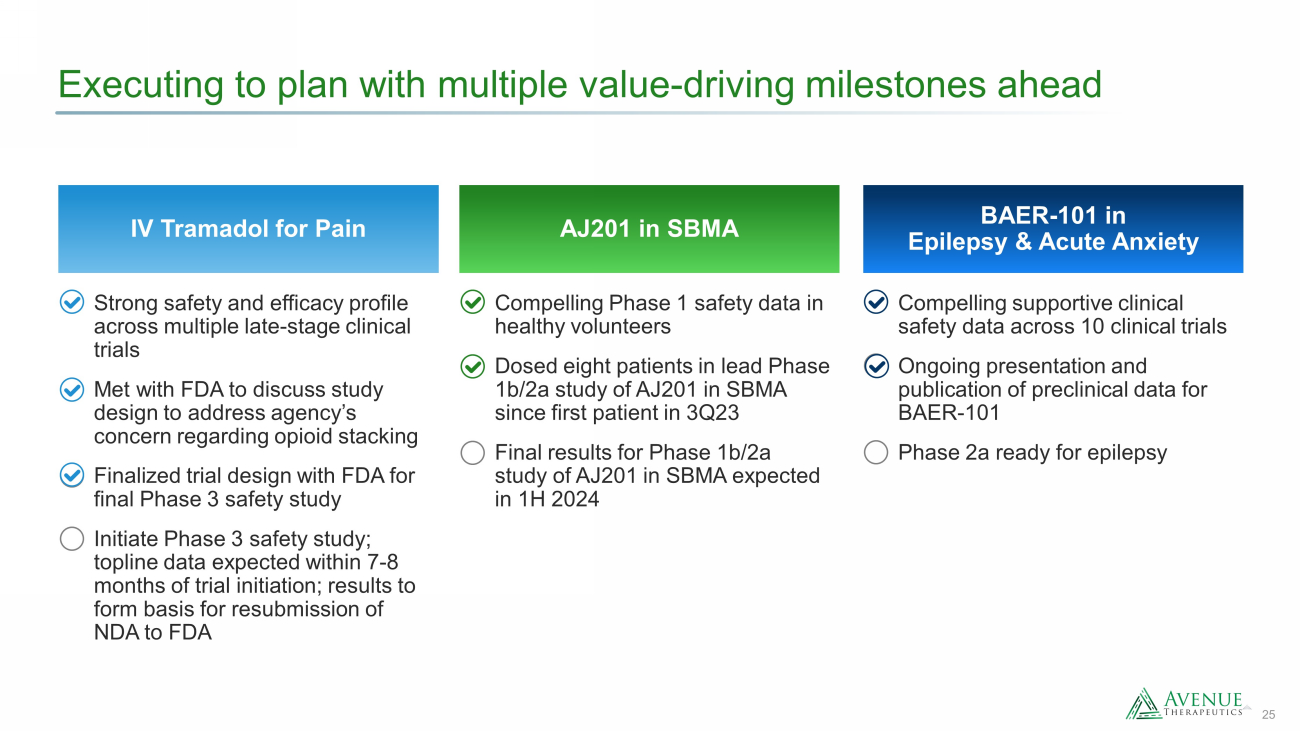
25 Executing to plan with multiple value - driving milestones ahead BAER - 101 in Epilepsy & Acute Anxiety AJ201 in SBMA Compelling Phase 1 safety data in healthy volunteers Dosed eight patients in lead Phase 1b/2a study of AJ201 in SBMA since first patient in 3Q23 Final results for Phase 1b/2a study of AJ201 in SBMA expected in 1H 2024 Compelling supportive clinical safety data across 10 clinical trials Ongoing presentation and publication of preclinical data for BAER - 101 Phase 2a ready for epilepsy IV Tramadol for Pain Strong safety and efficacy profile across multiple late - stage clinical trials Met with FDA to discuss study design to address agency’s concern regarding opioid stacking Finalized trial design with FDA for final Phase 3 safety study Initiate Phase 3 safety study; topline data expected within 7 - 8 months of trial initiation; results to form basis for resubmission of NDA to FDA

26 Led by experienced management team and board of directors Jay Kranzler MD PhD CEO, Urica Therapeutics Lindsay Rosenwald MD CEO, Fortress Biotech Neil Herskowitz Founder, ReGen Capital Curtis Oltmans Chief Legal Officer, Fulcrum Therapeutics Faith Charles Partner, Thompson Hine LLP Alexandra MacLean CEO, Avenue Therapeutics Management Board of Directors Alexandra MacLean MD CEO David Jin Interim CFO Michael Ryan VP Clinical Operations & Program Management

AVENUE THERAPEUTICS, INC. ⎸ NASDAQ: ATXI ⎸ OCTOBER 2023