
Exhibit 99.1

Avenue Therapeutics, Inc. Nasdaq: ATXI July 2017

Forward Looking Statements Statements in this presentation that are not descriptions of historical facts are forward - looking statements within the meaning of the “ safe harbor ” provisions of the Private Securities Litigation Reform Act of 1995. We have attempted to identify forward - looking statements by terminology including “ anticipates, ” “ believes, ” “ can, ” “ continue, ” “ could, ” “ estimates, ” “ expects, ” “ intends, ” “ may, ” “ plans, ” “ potential, ” “ predicts, ” “ should, ” or “ will ” or the negative of these terms or other comparable terminology. Forward - looking statements are based on management ’ s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and stock price. Factors that could cause actual results to differ materially from those currently anticipated are risks relating to: our growth strategy; results of research and development activities; uncertainties relating to preclinical and clinical testing; our dependence on third party suppliers; our ability to obtain, perform under and maintain financing and strategic agreements and relationships; our ability to attract, integrate, and retain key personnel; the early stage of products under development; our need for substantial funds; government regulation; patent and intellectual property matters; competition; as well as other risks described in the “Risk Factors” section of our Registration Statement on Form S - 1 initially filed with the Securities and Exchange Commission on April 28, 2017 as subsequently amended to date (our “Registration Statement”). We expressly disclaim any obligation or undertaking to update or revise any statements contained herein to reflect any change in our expectations or any changes in events, conditions or circumstances after the date of this presentation. You should read carefully our “Special Cautionary Notice Regarding Forward - looking Statements” and the factors described in the “Risk Factors” sections of our Registration Statement to better understand the risks and uncertainties inherent in our business. 2

Why IV Tramadol? • The only intravenous Schedule IV opioid in the U.S. if approved • Less addiction potential than widely prescribed narcotics in the hospital • Oral tramadol has established efficacy and safety • Physicians are already familiar with tramadol • Available “step - down” therapy and no need to switch to a different drug when patients go home • Reduced development risk • Entering Phase III in 3Q2017 • Phase III data in 2Q2018 3
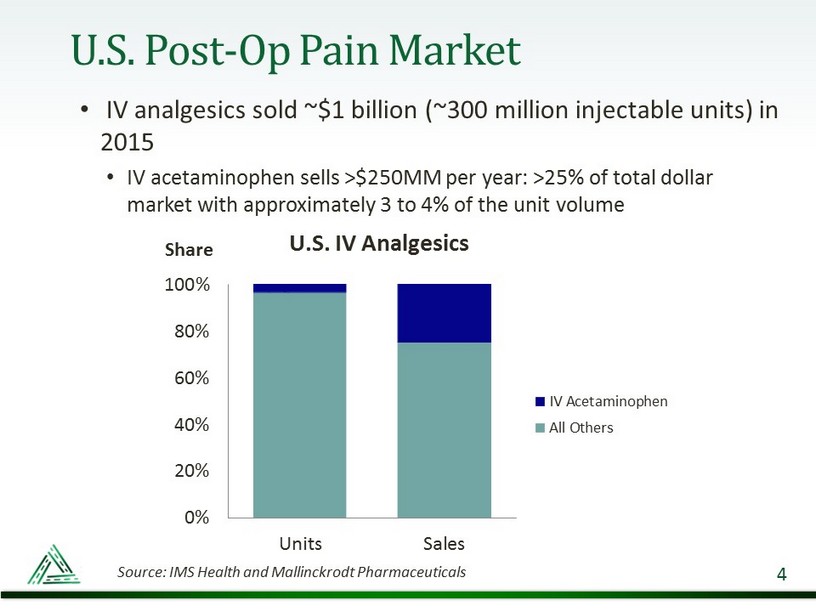
U.S. Post - Op Pain Market • IV analgesics sold ~$1 billion (~300 million injectable units) in 2015 • IV acetaminophen sells >$250MM per year: >25% of total dollar market with approximately 3 to 4% of the unit volume 4 0% 20% 40% 60% 80% 100% Units Sales U.S. IV Analgesics IV Acetaminophen All Others Share Source: IMS Health and Mallinckrodt Pharmaceuticals
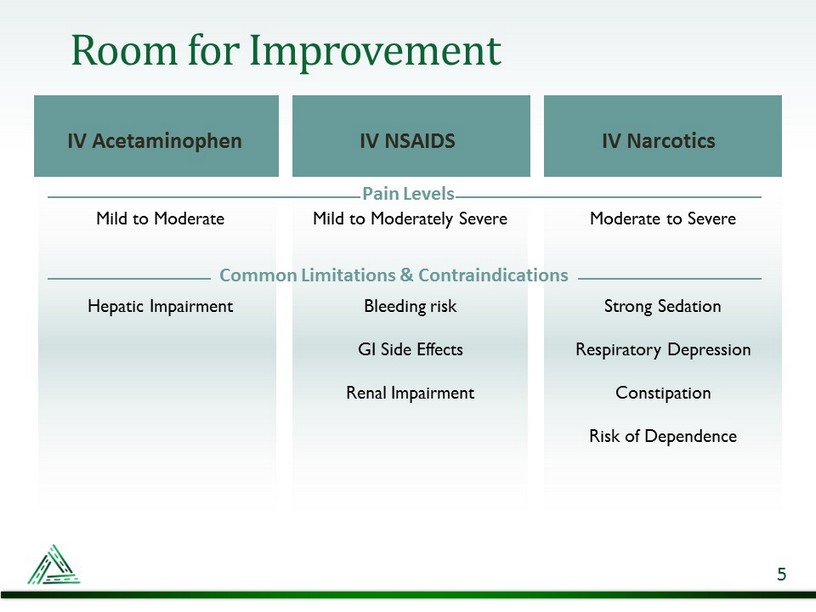
Mild to Moderate Hepatic Impairment Mild to Moderately Severe Bleeding risk GI Side Effects Renal Impairment Moderate to Severe Strong Sedation Respiratory Depression Constipation Risk of Dependence Room for Improvement IV Acetaminophen IV NSAIDS IV Narcotics Common Limitations & Contraindications Pain Levels 5
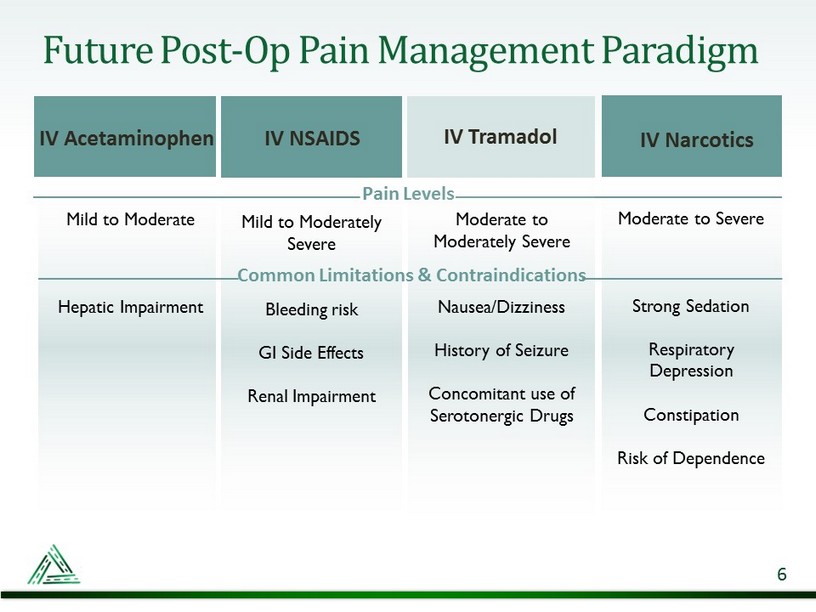
Mild to Moderate Hepatic Impairment Mild to Moderately Severe Bleeding risk GI Side Effects Renal Impairment Moderate to Severe Strong Sedation Respiratory Depression Constipation Risk of Dependence Future Post - Op Pain Management Paradigm IV Acetaminophen IV NSAIDS IV Narcotics Pain Levels 6 Moderate to Moderately Severe Nausea/Dizziness History of Seizure Concomitant use of Serotonergic Drugs Common Limitations & Contraindications IV Tramadol
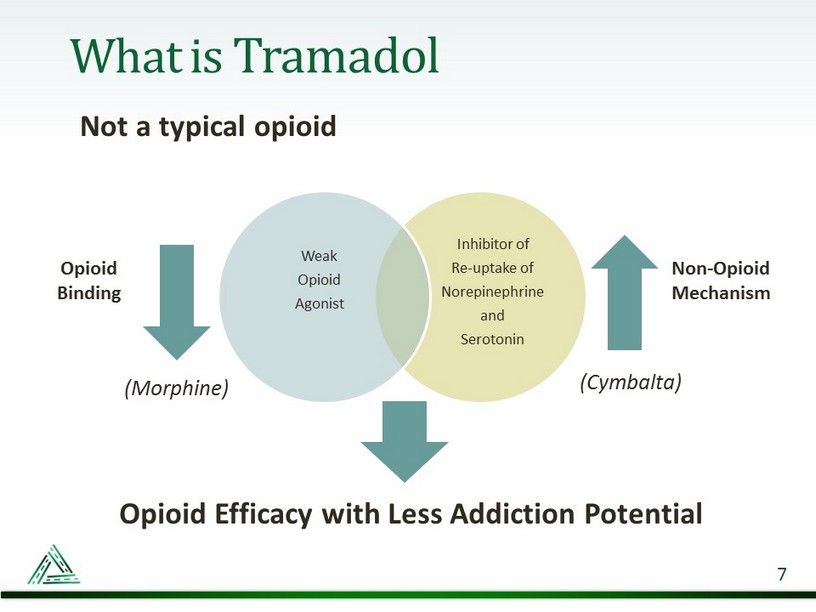
What is Tramadol Not a typical opioid 7 Inhibitor of Re - uptake of Norepinephrine and Serotonin Weak Opioid Agonist Opioid Binding Non - Opioid Mechanism Opioid Efficacy with Less Addiction Potential (Cymbalta) (Morphine)
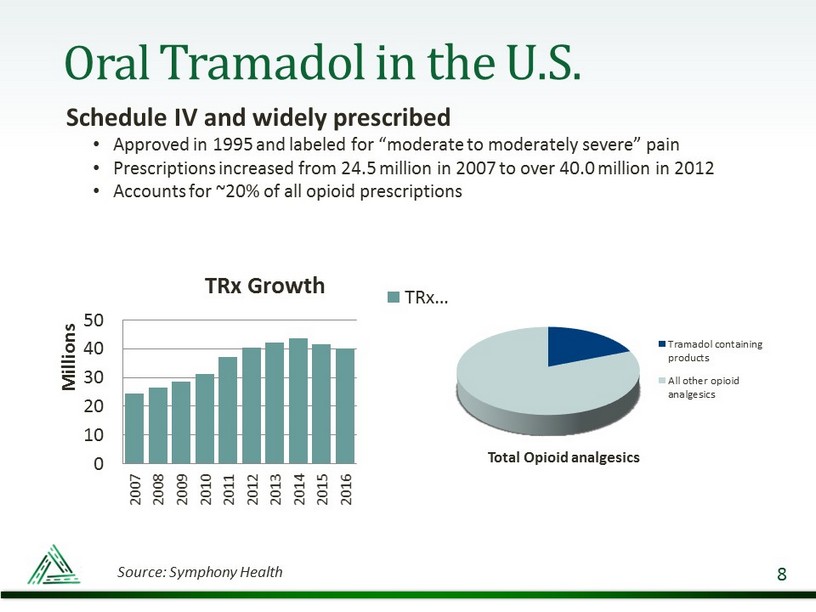
Oral Tramadol in the U.S. Schedule IV and widely prescribed • Approved in 1995 and labeled for “moderate to moderately severe” pain • Prescriptions increased from 24.5 million in 2007 to over 40.0 million in 2012 • Accounts for ~20% of all opioid prescriptions 8 0 10 20 30 40 50 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 Millions TRx Growth TRx… Total Opioid analgesics Tramadol containing products All other opioid analgesics Source: Symphony Health

Headlines on Opioid Crisis in the U.S. • After Record Year for Fatal Overdoses, New York City Targets Opioids – WSJ 3/13/2017 • Every 45 minutes, a child is poisoned by opioids – CNBC 3/20/2017 • FDA nominee says nation’s opioid crisis is as serious as Ebola, Zika threats – The Washington Post 4/5/2017 9
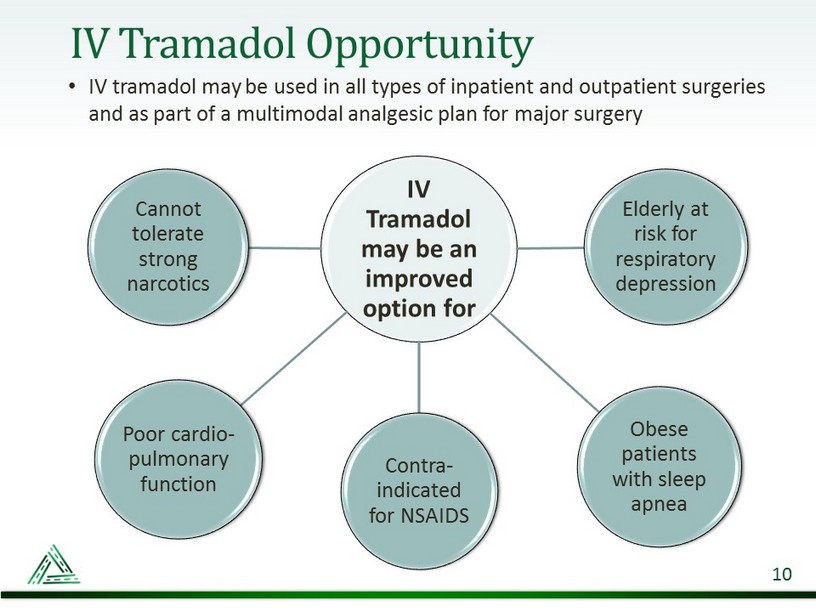
IV Tramadol Opportunity • IV tramadol may be used in all types of inpatient and outpatient surgeries and as part of a multimodal analgesic plan for major surgery 10 IV Tramadol may be an improved option for Contra - indicated for NSAIDS Elderly at risk for respiratory depression Obese patients with sleep apnea Poor cardio - pulmonary function Cannot tolerate strong narcotics
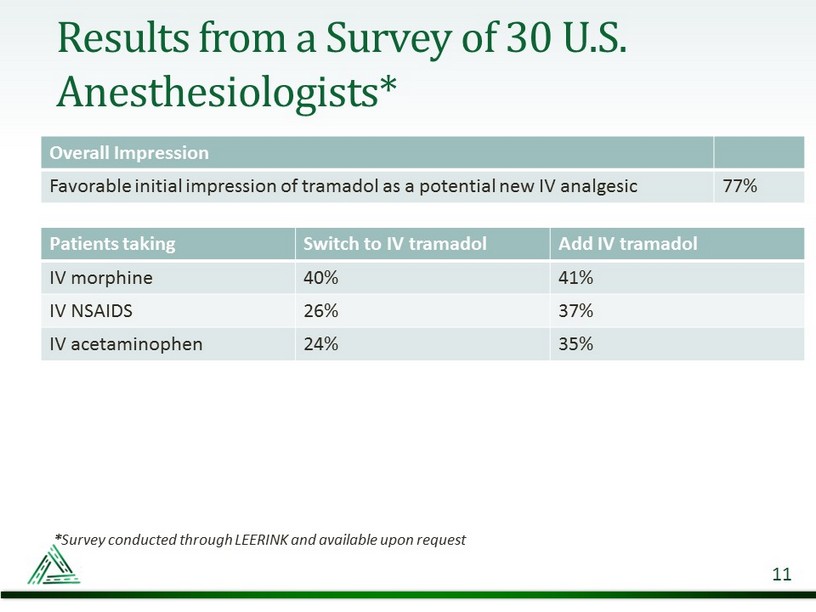
Results from a Survey of 30 U.S. Anesthesiologists* 11 * Survey conducted through LEERINK and available upon request Patients taking Switch to IV tramadol Add IV tramadol IV morphine 40% 41% IV NSAIDS 26% 37% IV acetaminophen 24% 35% Overall Impression Favorable initial impression of tramadol as a potential new IV analgesic 77%
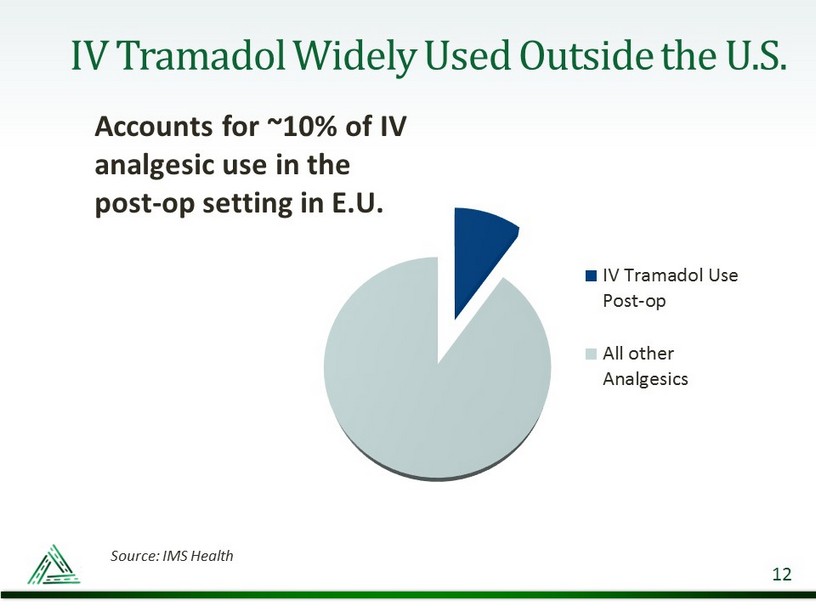
IV Tramadol Widely Used Outside the U.S. Accounts for ~10% of IV analgesic use in the post - op setting in E.U. 12 IV Tramadol Use Post-op All other Analgesics Source: IMS Health
Advantages of IV Tramadol • Oral tramadol has established efficacy and safety and physicians are familiar with it • Less addiction potential than widely prescribed narcotics in the hospital • Easily fitted into multi - modal pain management to avoid conventional opioids • Available “step - down” therapy • Patients can be transitioned from IV to oral tramadol 13
Novel Dosing Regimen • IV tramadol 50 mg is administered at Hours 0, 2, 4, and once every 4 hours thereafter • This dosing regimen was designed to provide a similar Cmax and AUC to that of 100 mg oral tramadol given every 6 hours at steady state 14
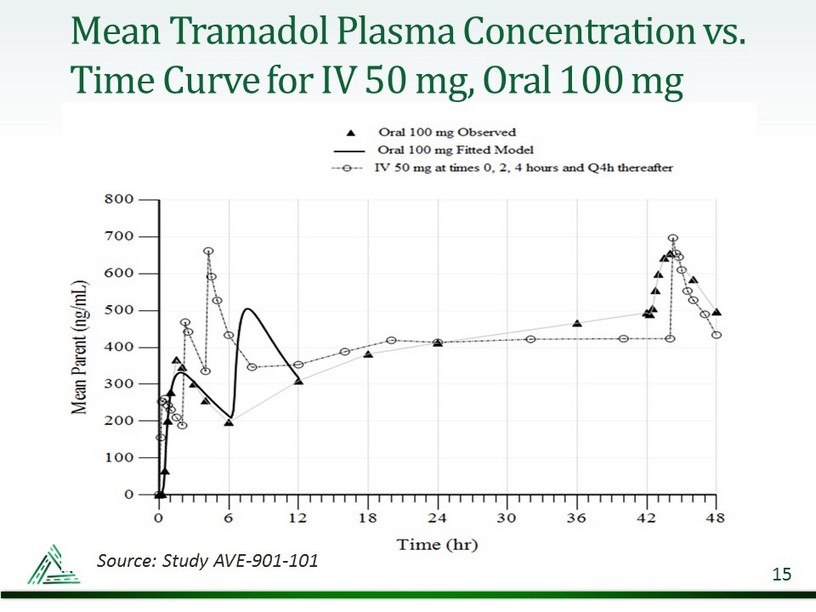
Mean Tramadol Plasma Concentration vs. Time Curve for IV 50 mg, Oral 100 mg 15 Source: Study AVE - 901 - 101
Intellectual Property • Strong patent portfolio on Intravenous Administration of Tramadol • U.S. Patents No. 8,895,622, No. 9,561,195 and No. 9,566,253 • U.S. Patent No. 9,693,949 • Further Patent Applications are contemplated 16
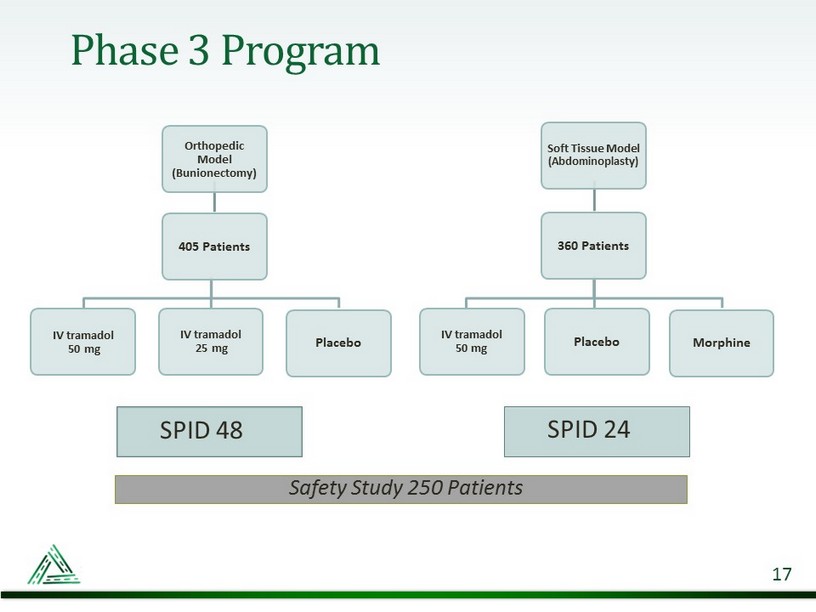
Phase 3 Program 17 Orthopedic Model (Bunionectomy) 405 Patients IV tramadol 50 mg IV tramadol 25 mg Placebo Soft Tissue Model (Abdominoplasty) 360 Patients IV tramadol 50 mg Placebo Morphine SPID 48 SPID 24 Safety Study 250 Patients
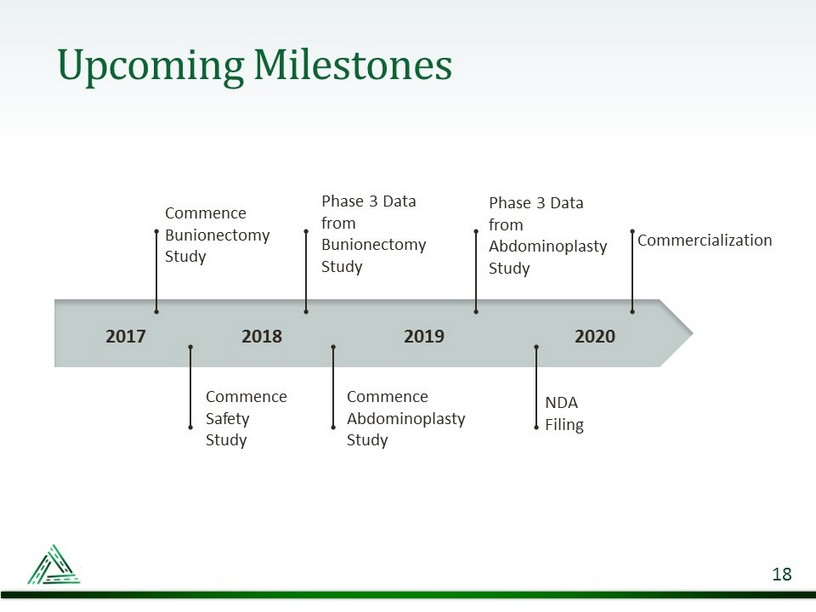
Upcoming Milestones 18 2017 2018 2019 Commence Bunionectomy Study Commence Safety Study Commence Abdominoplasty Study Commercialization 2020 Phase 3 Data from Bunionectomy Study NDA Filing Phase 3 Data from Abdominoplasty Study
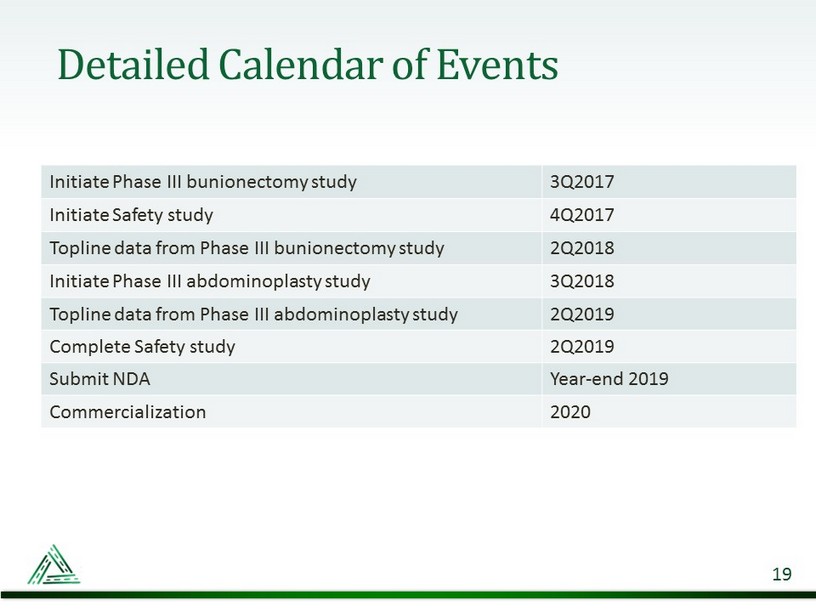
Detailed Calendar of Events 19 Initiate Phase III bunionectomy study 3Q2017 Initiate Safety study 4Q2017 Topline data from Phase III bunionectomy study 2Q2018 Initiate Phase III abdominoplasty study 3Q2018 Topline data from Phase III abdominoplasty study 2Q2019 Complete Safety study 2Q2019 Submit NDA Year - end 2019 Commercialization 2020

Lucy Lu, M.D. President and Chief Executive Officer Avenue Therapeutics, Inc. 2 Gansevoort St., 9 th Floor New York, NY 10014 Tel: 781 - 652 - 4500/Email: ir@avenuetx.com 20 Avenue Therapeutics, Inc.