
Exhibit 99.1

AVENUE THERAPEUTICS, INC. ⎸ NASDAQ: ATXI ⎸ MAY 2018

Forward Looking Statements Statements in this presentation that are not descriptions of historical facts are forward - looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. We have attempted to identify forward - looking statements by terminology including “anticipates,” “believes,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “should,” or “will” or the negative of these terms or other comparable terminology. Forward - looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and stock price. Factors that could cause actual results to differ materially from those currently anticipated are risks relating to: our growth strategy; results of research and development activities; uncertainties relating to preclinical and clinical testing; our dependence on third party suppliers; our ability to obtain, perform under and maintain financing and strategic agreements and relationships; our ability to attract, integrate, and retain key personnel; the early stage of products under development; our need for substantial funds; government regulation; patent and intellectual property matters; competition; as well as other risks described in the “Risk Factors” section of our Annual Report on Form 10 - K for the year ended December 31, 2017 (“Form 10 - K”) and other periodic reports filed from time to time with the Securities and Exchange Commission. We expressly disclaim any obligation or undertaking to update or revise any statements contained herein to reflect any change in our expectations or any changes in events, conditions or circumstances after the date of this presentation. You should read carefully our “Special Cautionary Notice Regarding Forward - looking Statements” and the factors described in the “Risk Factors” sections of our Form 10 - K and other periodic reports to better understand the risks and uncertainties inherent in our business.
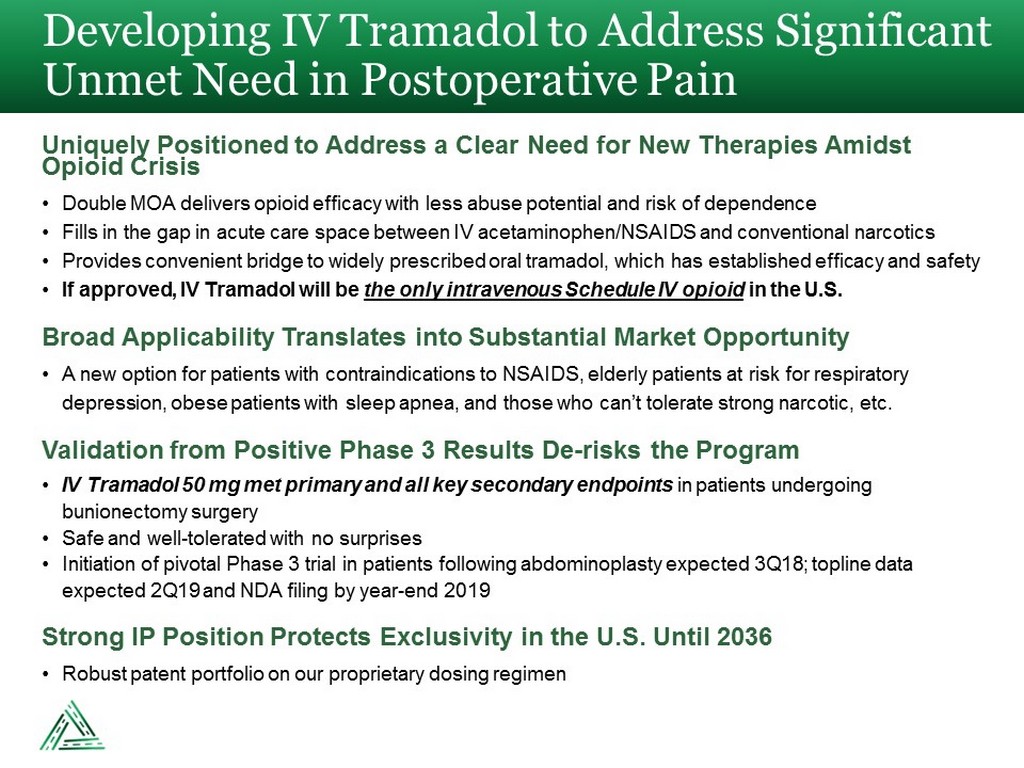
Developing IV Tramadol to Address Significant Unmet Need in Postoperative Pain Uniquely Positioned to Address a Clear Need for New Therapies Amidst Opioid Crisis • Double MOA delivers opioid efficacy with less abuse potential and risk of dependence • Fills in the gap in acute care space between IV acetaminophen/NSAIDS and conventional narcotics • Provides convenient bridge to widely prescribed oral tramadol, which has established efficacy and safety • If approved, IV Tramadol will be the only intravenous Schedule IV opioid in the U.S. Broad Applicability Translates into Substantial Market Opportunity • A new option for patients with contraindications to NSAIDS, elderly patients at risk for respiratory depression, obese patients with sleep apnea, and those who can’t tolerate strong narcotic, etc. Validation from Positive Phase 3 Results De - risks the Program • IV Tramadol 50 mg met primary and all key secondary endpoints in patients undergoing bunionectomy surgery • Safe and well - tolerated with no surprises • Initiation of pivotal Phase 3 trial in patients following abdominoplasty expected 3Q18; topline data expected 2Q19 and NDA filing by year - end 2019 Strong IP Position Protects Exclusivity in the U.S. Until 2036 • Robust patent portfolio on our proprietary dosing regimen
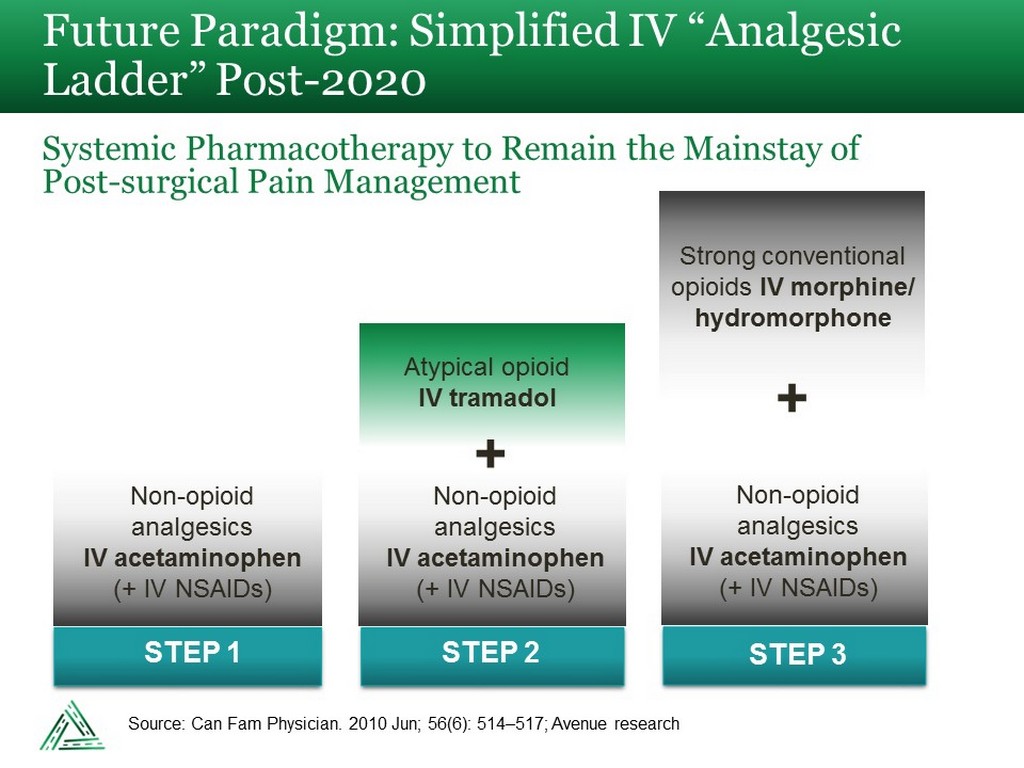
Future Paradigm: Simplified IV “Analgesic Ladder” Post - 2020 Systemic Pharmacotherapy to Remain the Mainstay of Post - surgical Pain Management STEP 1 STEP 2 Non - opioid analgesics IV acetaminophen (+ IV NSAIDs) Atypical opioid IV tramadol + + Strong conventional opioids IV morphine/ hydromorphone STEP 3 Non - opioid analgesics IV acetaminophen (+ IV NSAIDs) Non - opioid analgesics IV acetaminophen (+ IV NSAIDs) Source: Can Fam Physician. 2010 Jun; 56(6): 514 – 517; Avenue research
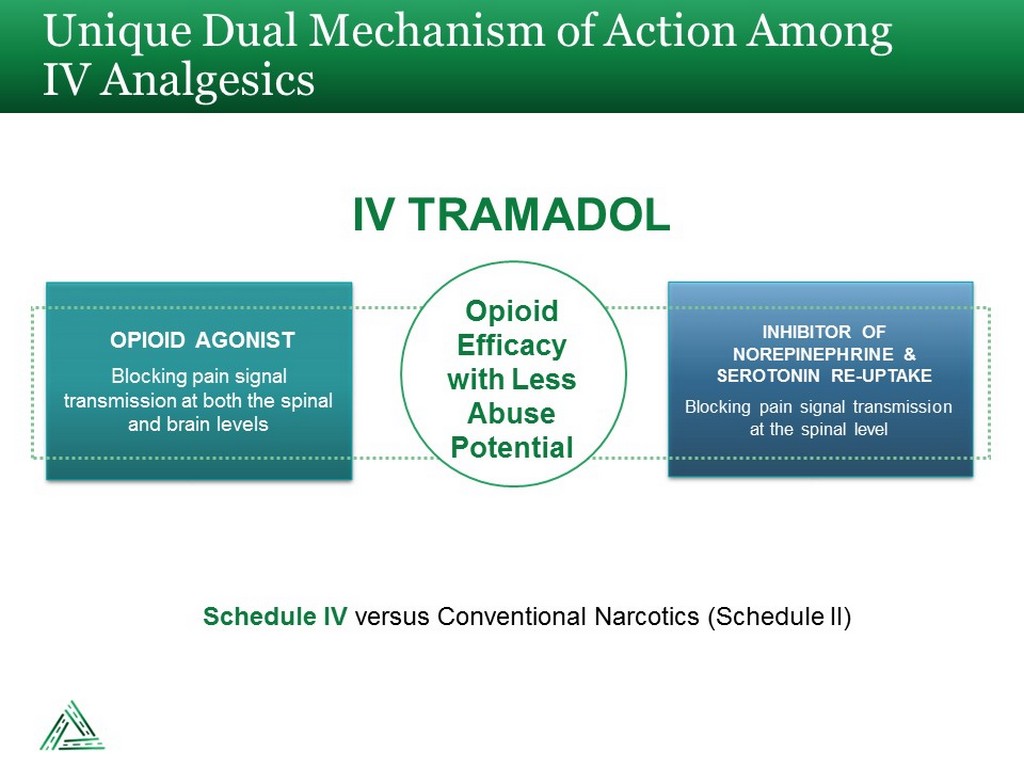
Unique Dual Mechanism of Action Among IV Analgesics IV TRAMADOL Blocking pain signal transmission at both the spinal and brain levels OPIOID AGONIST Blocking pain signal transmission at the spinal level INHIBITOR OF NOREPINEPHRINE & SEROTONIN RE - UPTAKE Opioid Efficacy with Less Abuse Potential Schedule IV versus Conventional Narcotics (Schedule II)
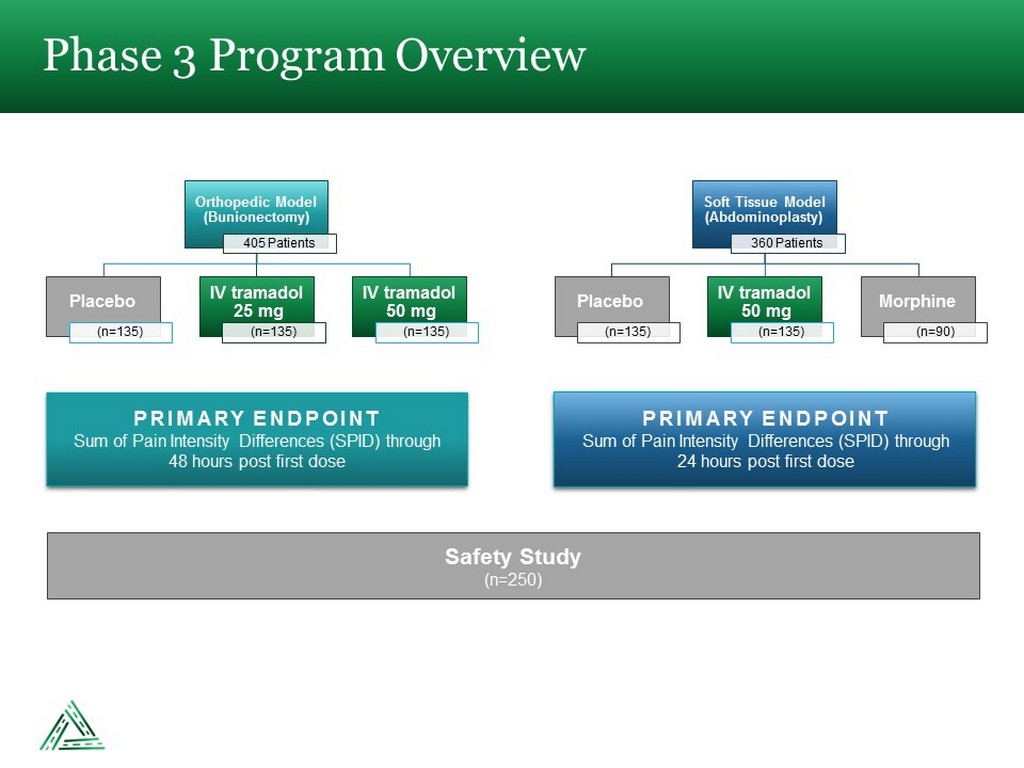
Phase 3 Program Overview Orthopedic Model (Bunionectomy) 405 Patients Placebo (n=135) IV tramadol 25 mg (n=135) IV tramadol 50 mg (n=135) Soft Tissue Model (Abdominoplasty) 360 Patients Placebo (n=135) IV tramadol 50 mg (n=135) Morphine (n=90) PRIMARY ENDPOINT Sum of Pain Intensity Differences (SPID) through 48 hours post first dose PRIMARY ENDPOINT Sum of Pain Intensity Differences (SPID) through 24 hours post first dose Safety Study (n=250)
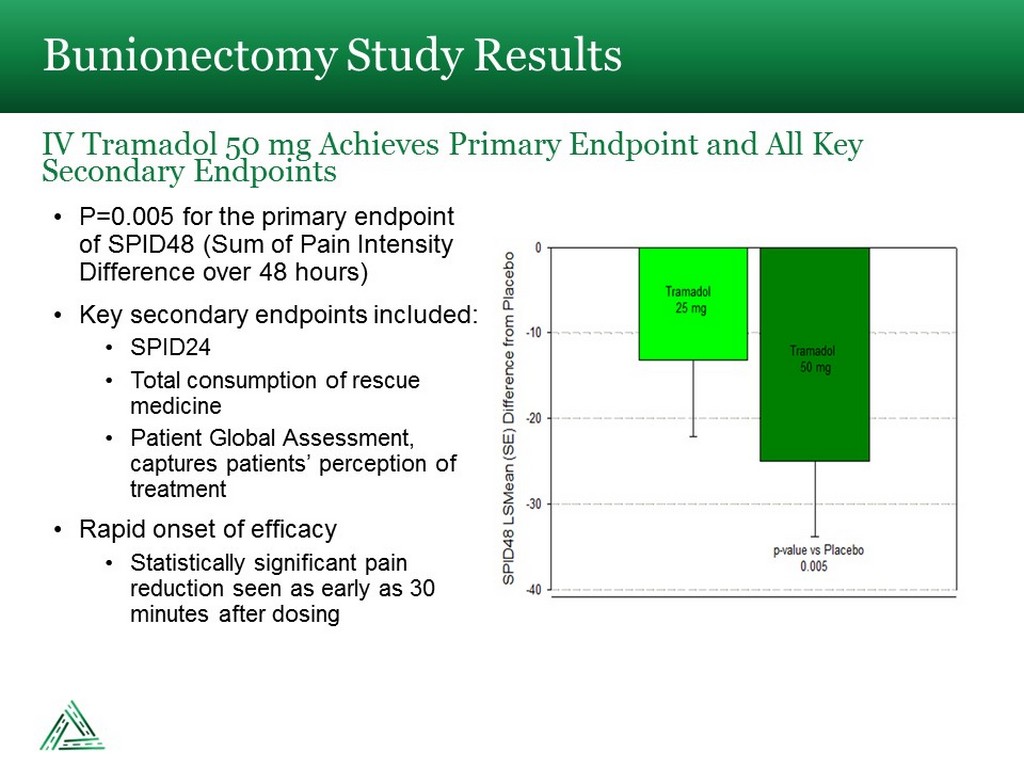
• P=0.005 for the primary endpoint of SPID48 (Sum of Pain Intensity Difference over 48 hours) • Key secondary endpoints included: • SPID24 • Total consumption of rescue medicine • Patient Global Assessment, captures patients’ perception of treatment • Rapid onset of efficacy • Statistically significant pain reduction seen as early as 30 minutes after dosing Bunionectomy Study Results IV Tramadol 50 mg Achieves Primary Endpoint and All Key Secondary Endpoints
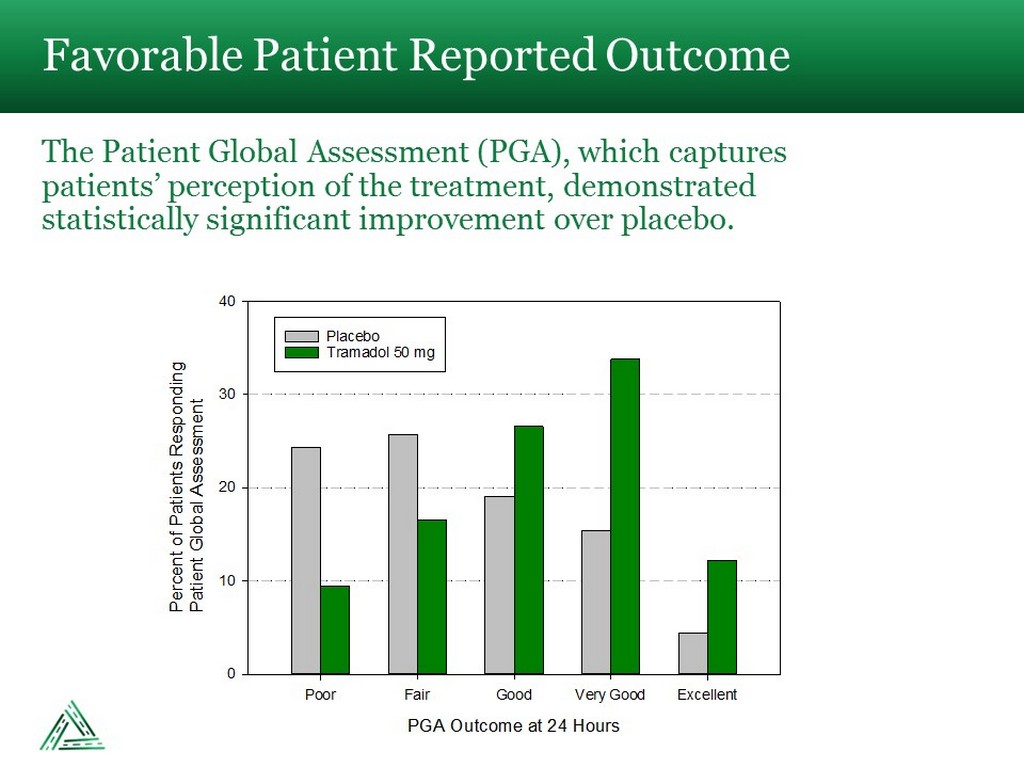
Favorable Patient Reported Outcome The Patient Global Assessment (PGA), which captures patients’ perception of the treatment, demonstrated statistically significant improvement over placebo. PGA Outcome at 24 Hours Poor Fair Good Very Good Excellent Percent of Patients RespondingPatient Global Assessment 0 10 20 30 40 Placebo Tramadol 50 mg
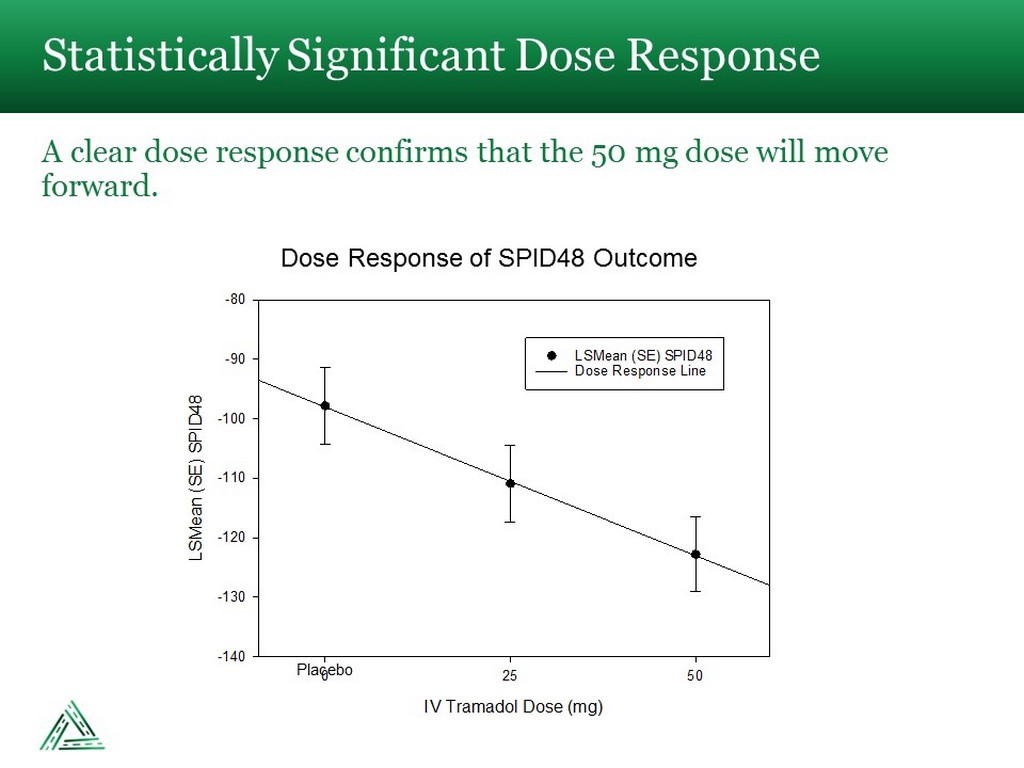
Statistically Significant Dose Response A clear dose response confirms that the 50 mg dose will move forward. IV Tramadol Dose (mg) 0 25 50 LSMean (SE) SPID48 -140 -130 -120 -110 -100 -90 -80 LSMean (SE) SPID48 Dose Response Line Placebo Dose Response of SPID48 Outcome

• There were no drug - related serious adverse events (SAEs) • The most common (≥5%) adverse events in the trial where IV tramadol 50 mg differed from placebo were nausea, vomiting, dizziness and somnolence. • Most of these adverse events were mild or moderate (Grade 1 or 2) with only 4 (3%) patients experiencing a Grade 3 event (vomiting) and no Grade 4 events in the IV tramadol 50 mg group • One patient in the 50 mg IV tramadol arm discontinued from the study due to adverse events. • IV Tramadol 50 mg patients were able to complete the study and receive all their treatment doses • Only 2 (1.4%) IV Tramadol 50 mg patients discontinued the study, versus 16 (11.8%) placebo patients • 98.6% of IV Tramadol 50 mg patients received their full course of infusions during the study No Surprise in Safety Outcomes IV Tramadol 50 mg Was Well Tolerated
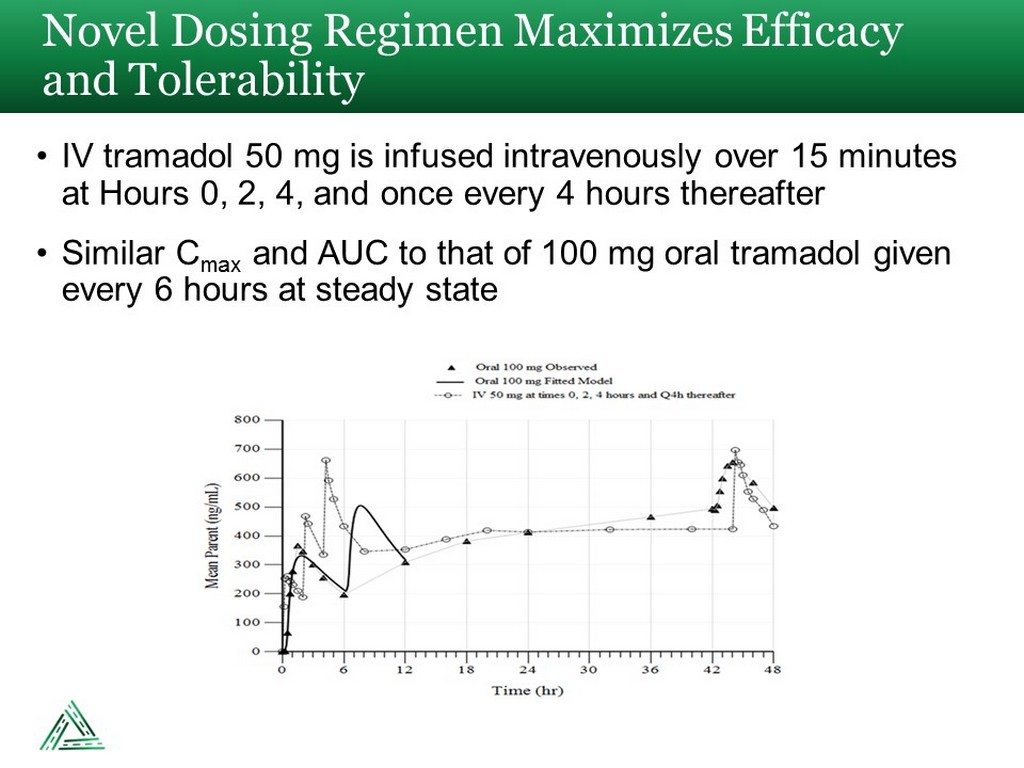
• IV tramadol 50 mg is infused intravenously over 15 minutes at Hours 0, 2, 4, and once every 4 hours thereafter • Similar C max and AUC to that of 100 mg oral tramadol given every 6 hours at steady state Novel Dosing Regimen Maximizes Efficacy and Tolerability
Post - Surgical Pain Management is a Gateway to Opioid Dependence Approximately 6% of patients become new persistent opioid users in the post - surgical setting “In this population - based study of 36,177 surgical patients, the incidence of new persistent opioid use after surgical procedures was 5.9% to 6.5% and did not differ between major and minor surgical procedures.” (1) “After adjusting for covariates, other risk factors… including benzodiazepines …as well as regimens initiated with hydromorphone… and oxycodone …had a statistically significant association with opioid misuse.” (2) Regimens initiated with conventional narcotics have a significant association with opioid misuse (1) Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017;152(6):e170504. (2) Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naïve patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018:Jan 17;360:j5790.
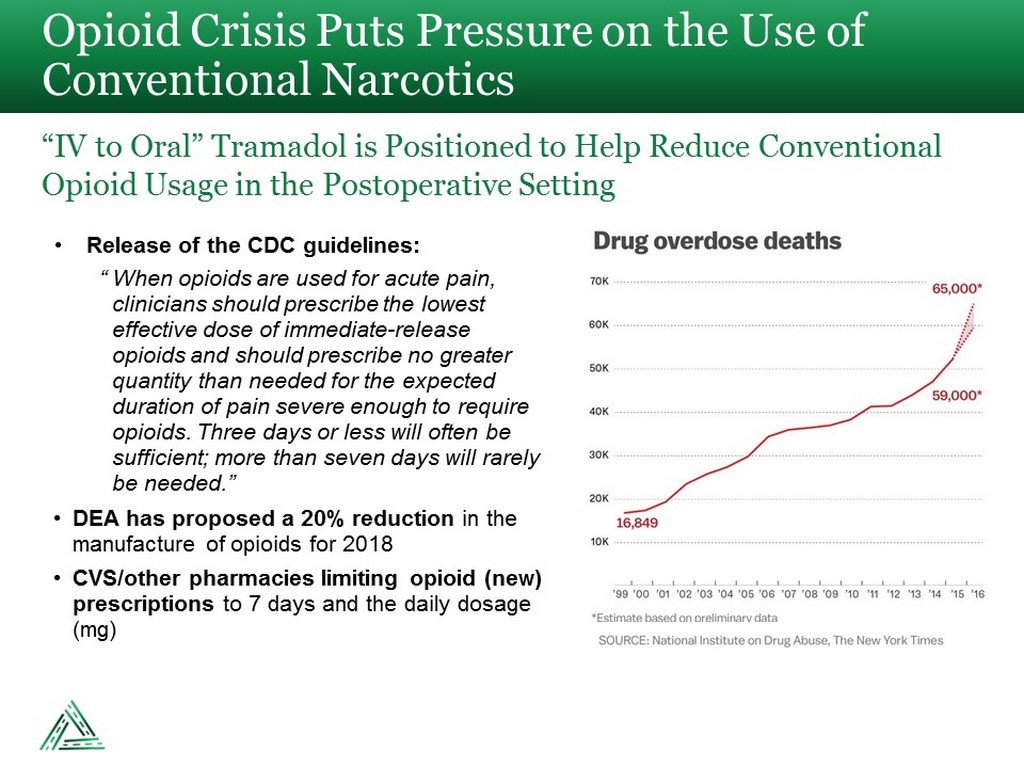
• Release of the CDC guidelines: “ When opioids are used for acute pain, clinicians should prescribe the lowest effective dose of immediate - release opioids and should prescribe no greater quantity than needed for the expected duration of pain severe enough to require opioids. Three days or less will often be sufficient; more than seven days will rarely be needed.” • DEA has proposed a 20% reduction in the manufacture of opioids for 2018 • CVS/other pharmacies limiting opioid (new) prescriptions to 7 days and the daily dosage (mg) Opioid Crisis Puts Pressure on the Use of Conventional Narcotics “IV to Oral” Tramadol is Positioned to Help Reduce Conventional Opioid Usage in the Postoperative Setting
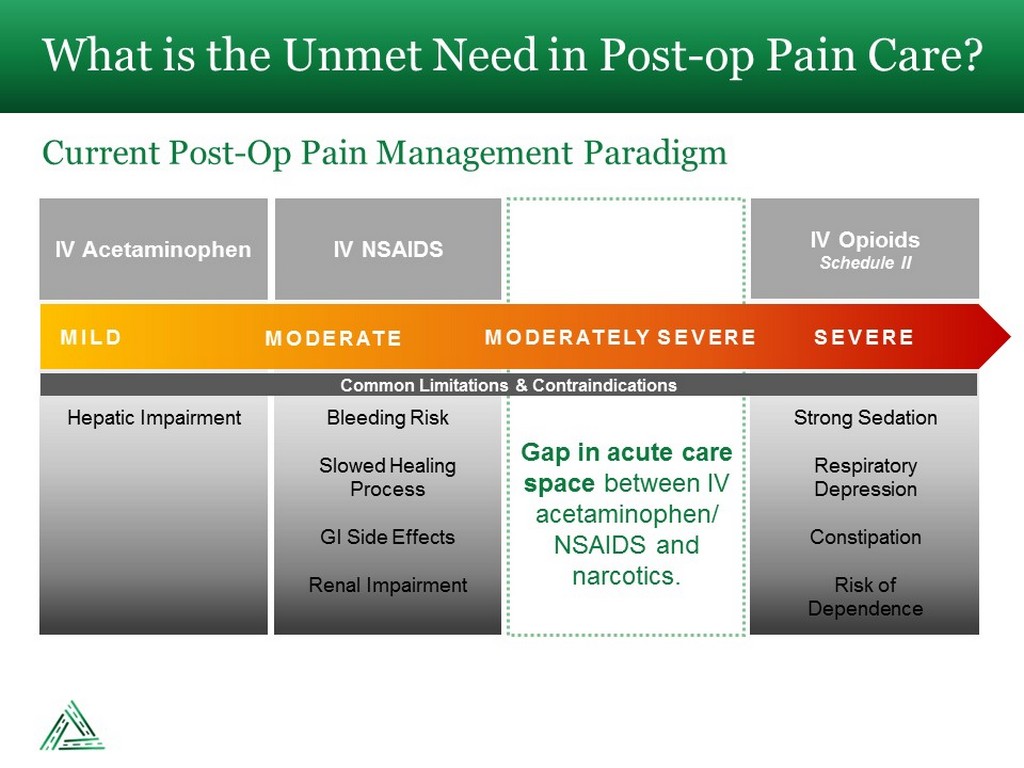
What is the Unmet Need in Post - op Pain Care? Current Post - Op Pain Management Paradigm IV Acetaminophen IV NSAIDS IV Opioids Schedule II MILD MODERATE MODERATELY SEVERE SEVERE Common Limitations & Contraindications Hepatic Impairment Bleeding Risk Slowed Healing Process GI Side Effects Renal Impairment Strong Sedation Respiratory Depression Constipation Risk of Dependence Gap in acute care space between IV acetaminophen/ NSAIDS and narcotics.
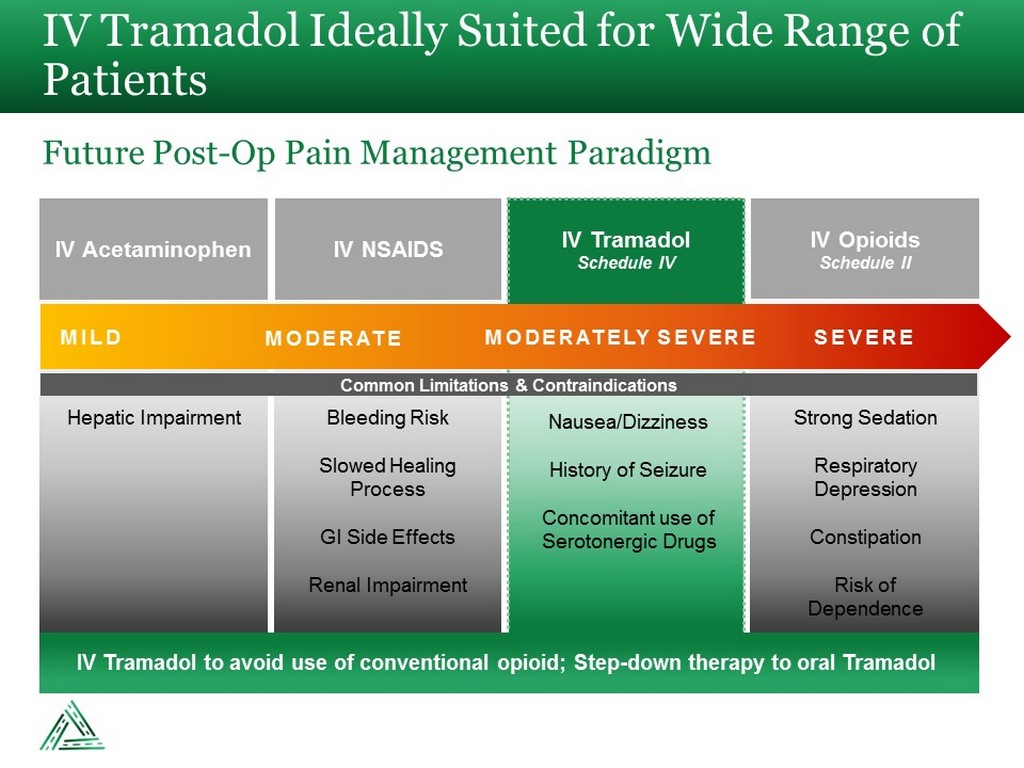
IV Tramadol Ideally Suited for Wide Range of Patients Future Post - Op Pain Management Paradigm IV Acetaminophen IV NSAIDS IV Opioids Schedule II IV Tramadol Schedule IV MILD MODERATE MODERATELY SEVERE SEVERE Common Limitations & Contraindications Hepatic Impairment Bleeding Risk Slowed Healing Process GI Side Effects Renal Impairment Nausea/Dizziness History of Seizure Concomitant use of Serotonergic Drugs Strong Sedation Respiratory Depression Constipation Risk of Dependence IV Tramadol to avoid use of conventional opioid; Step - down therapy to oral Tramadol
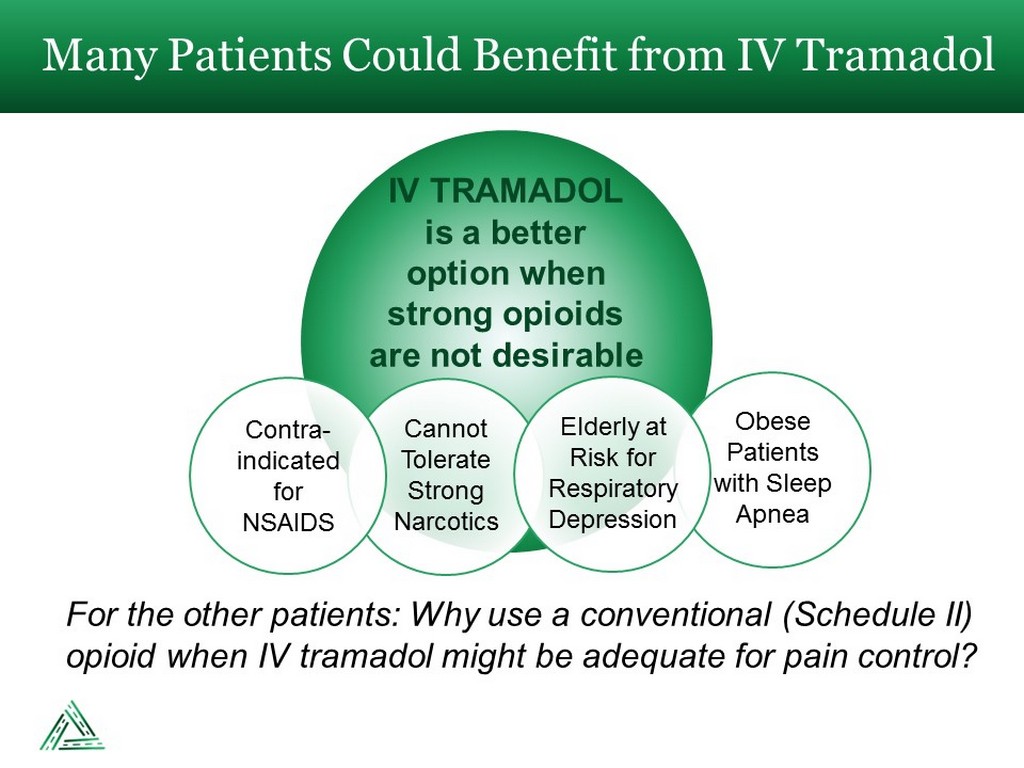
IV TRAMADOL is a better option when strong opioids are not desirable Many Patients Could Benefit from IV Tramadol Cannot Tolerate Strong Narcotics Contra - indicated for NSAIDS Obese Patients with Sleep Apnea Elderly at Risk for Respiratory Depression For the other patients: Why use a conventional (Schedule II) opioid when IV tramadol might be adequate for pain control?
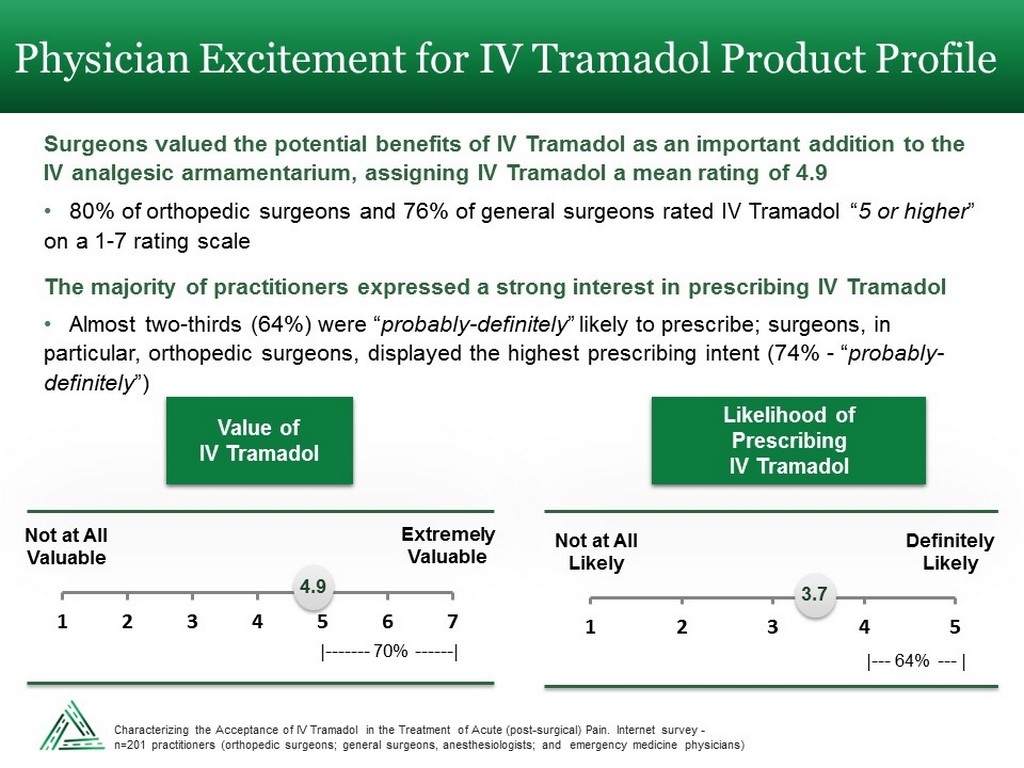
Physician Excitement for IV Tramadol Product Profile Surgeons valued the potential benefits of IV Tramadol as an important addition to the IV analgesic armamentarium, assigning IV Tramadol a mean rating of 4.9 • 80% of orthopedic surgeons and 76% of general surgeons rated IV Tramadol “ 5 or higher ” on a 1 - 7 rating scale The majority of practitioners expressed a strong interest in prescribing IV Tramadol • Almost two - thirds (64%) were “ probably - definitely ” likely to prescribe; surgeons, in particular, orthopedic surgeons, displayed the highest prescribing intent (74% - “ probably - definitely ”) 1 2 3 4 5 6 7 4.9 Not at All Valuable Extremely Valuable Value of IV Tramadol | ------- 70% ------ | 1 2 3 4 5 3.7 Not at All Likely Definitely Likely Likelihood of Prescribing IV Tramadol | --- 64% --- | Characterizing the Acceptance of IV Tramadol in the Treatment of Acute (post - surgical) Pain. Internet survey - n=201 practitioners (orthopedic surgeons; general surgeons, anesthesiologists; and emergency medicine physicians)
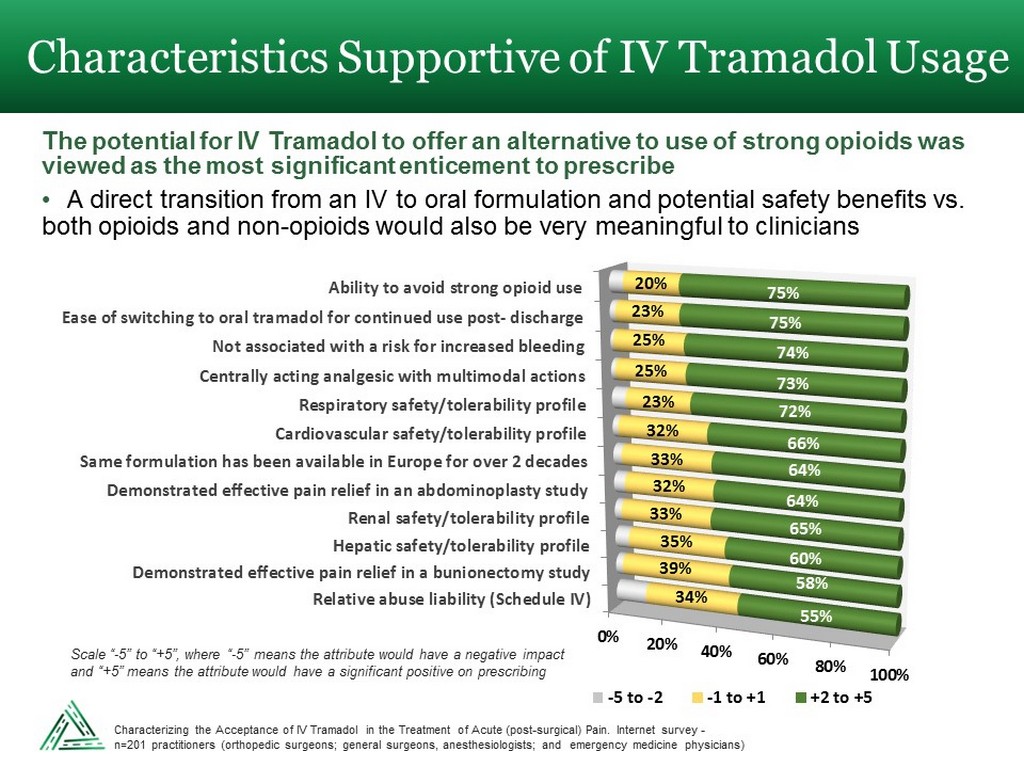
Characteristics Supportive of IV Tramadol Usage 0% 20% 40% 60% 80% 100% Relative abuse liability (Schedule IV) Demonstrated effective pain relief in a bunionectomy study Hepatic safety/tolerability profile Renal safety/tolerability profile Demonstrated effective pain relief in an abdominoplasty study Same formulation has been available in Europe for over 2 decades Cardiovascular safety/tolerability profile Respiratory safety/tolerability profile Centrally acting analgesic with multimodal actions Not associated with a risk for increased bleeding Ease of switching to oral tramadol for continued use post- discharge Ability to avoid strong opioid use 34% 39% 35% 33% 32% 33% 32% 23% 25% 25% 23% 20% 55% 58% 60% 65% 64% 64% 66% 72% 73% 74% 75% 75% -5 to -2 -1 to +1 +2 to +5 The potential for IV Tramadol to offer an alternative to use of strong opioids was viewed as the most significant enticement to prescribe • A direct transition from an IV to oral formulation and potential safety benefits vs. both opioids and non - opioids would also be very meaningful to clinicians Scale “ - 5” to “+5”, where “ - 5” means the attribute would have a negative impact and “+5” means the attribute would have a significant positive on prescribing Characterizing the Acceptance of IV Tramadol in the Treatment of Acute (post - surgical) Pain. Internet survey - n=201 practitioners (orthopedic surgeons; general surgeons, anesthesiologists; and emergency medicine physicians)
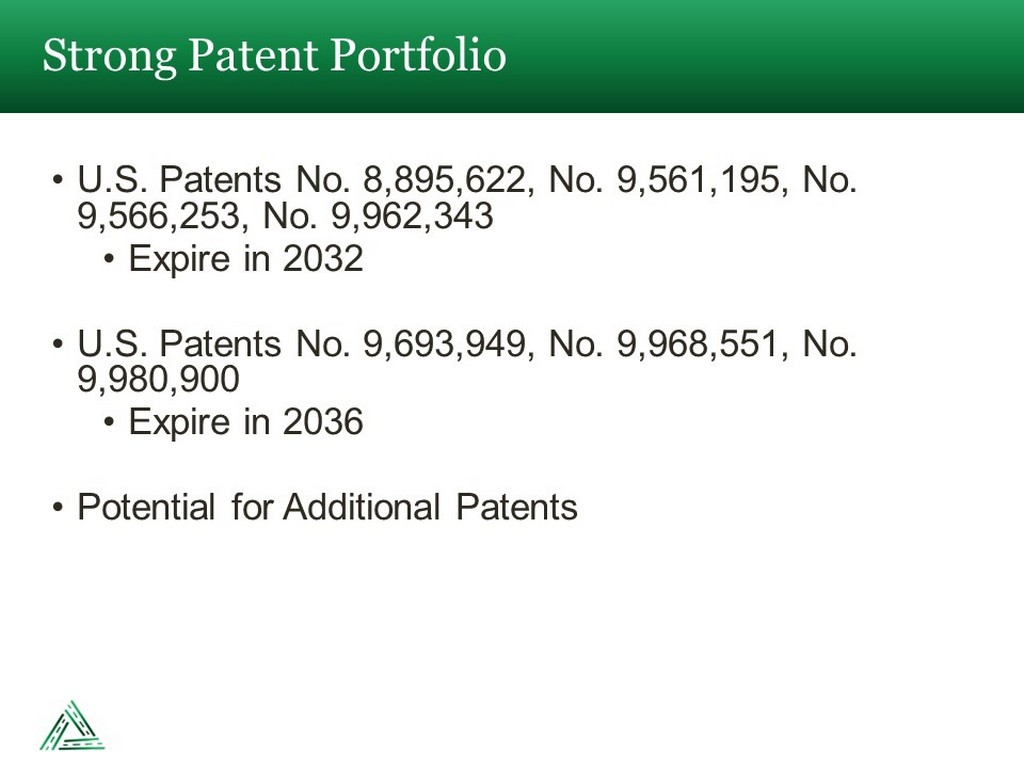
• U.S. Patents No. 8,895,622, No. 9,561,195, No. 9,566,253, No. 9,962,343 • Expire in 2032 • U.S. Patents No. 9,693,949, No. 9,968,551, No. 9,980,900 • Expire in 2036 • Potential for Additional Patents Strong Patent Portfolio

• Licensed drug from Bristol - Myers Squibb in 2006 – upfront fee of $25 million and $15 million milestone upon FDA approval • FDA approval in November 2010 and launched in January 2011 • List price of $14.75 per vial as of 2013 • Royalties in mid - teens to mid - twenties • Acquired on February 11, 2014 for $1.3 billion by Mallinckrodt • Expected authorized generic launch in December 2020 A Prior Success Story – IV Acetaminophen Cadence Pharmaceuticals - OFIRMEV ® 0 20 40 60 80 100 120 2011 2012 2013 Sales (in $ Millions) $111M $50M $12M Sales:
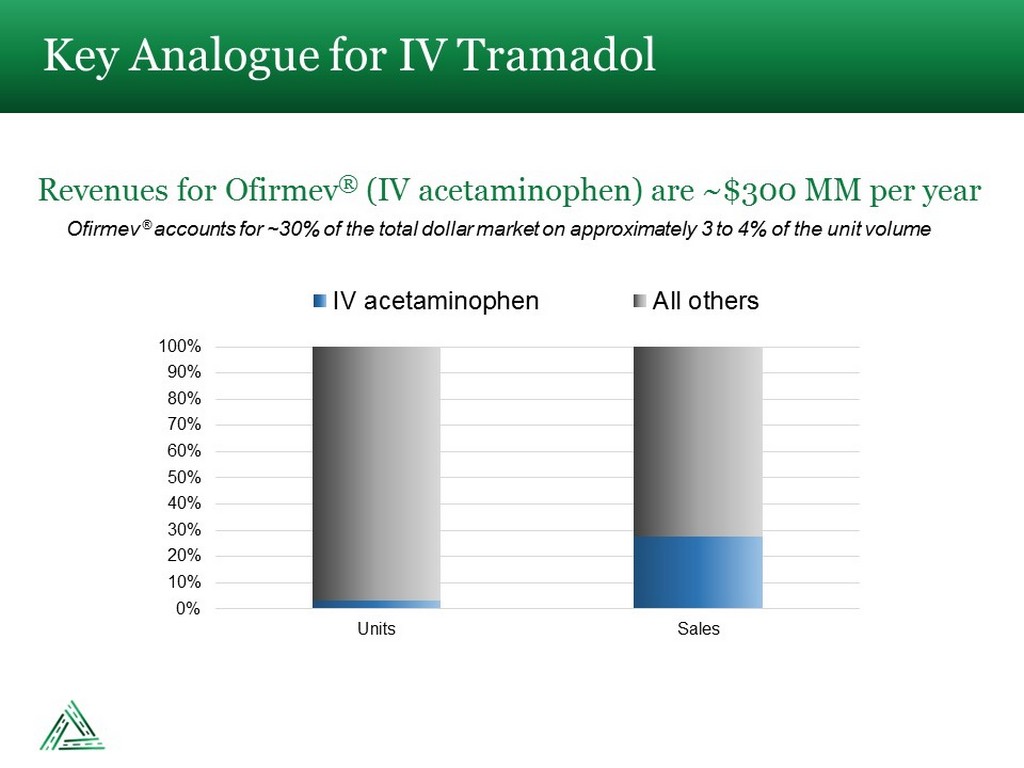
Ofirmev ® accounts for ~30% of the total dollar market on approximately 3 to 4% of the unit volume Key Analogue for IV Tramadol Revenues for Ofirmev ® (IV acetaminophen) are ~$300 MM per year 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Units Sales IV acetaminophen All others
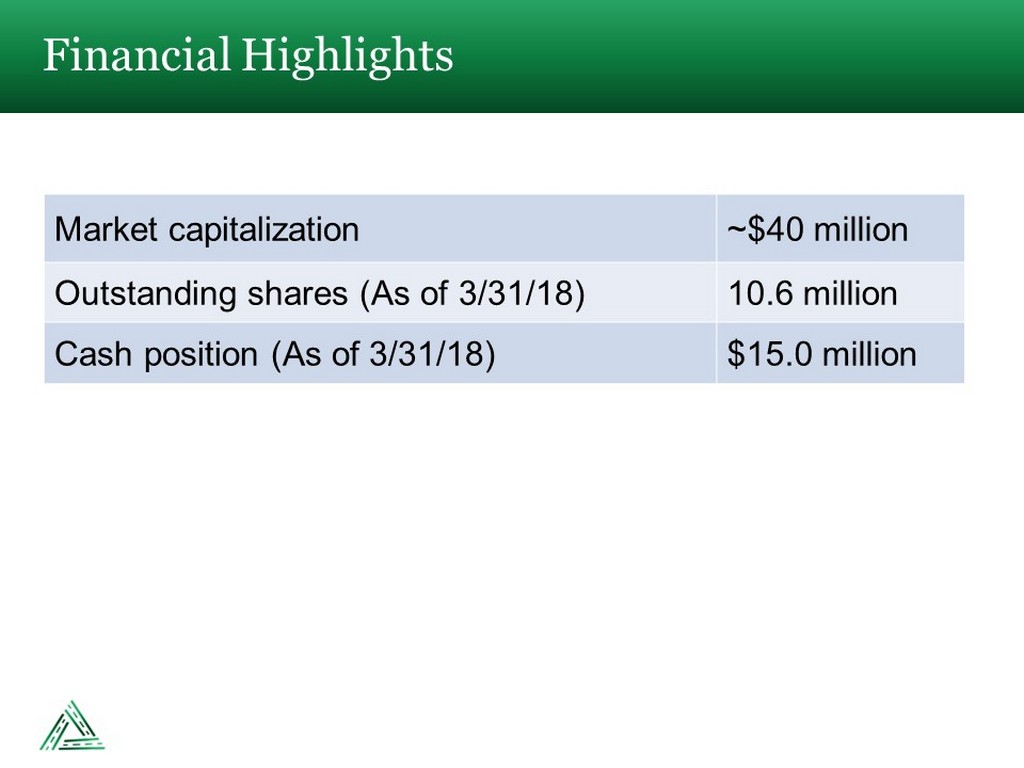
Financial Highlights Market capitalization ~$40 million Outstanding shares (As of 3/31/18) 10.6 million Cash position (As of 3/31/18) $15.0 million
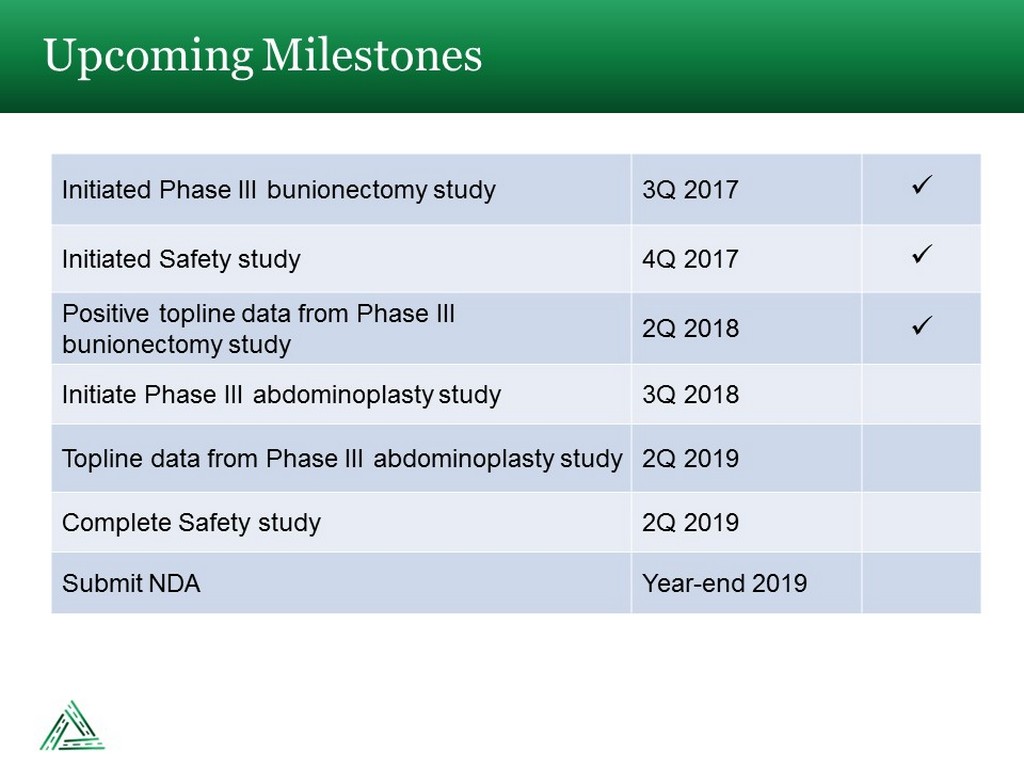
Upcoming Milestones Initiated Phase III bunionectomy study 3Q 2017 x Initiated Safety study 4Q 2017 x Positive topline data from Phase III bunionectomy study 2Q 2018 x Initiate Phase III abdominoplasty study 3Q 2018 Topline data from Phase III abdominoplasty study 2Q 2019 Complete Safety study 2Q 2019 Submit NDA Year - end 2019

AVENUE THERAPEUTICS, INC. ⎸ NASDAQ: ATXI ⎸ MAY 2018